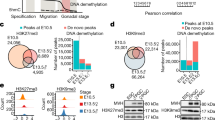
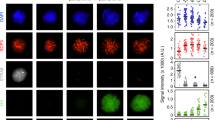
Abstract
Germ cells have the unique capacity to start a new life upon fertilization. They are generated during a sex-specific differentiation programme called gametogenesis. Maturation of germ cells is characterized by an impressive degree of cellular restructuring and gene regulation that involves remarkable genomic reorganization. These events are finely tuned, but are also susceptible to the introduction of various types of error. Because stable genetic transmission to future generations is essential for life, understanding the control of these processes has far-reaching implications for human health and reproduction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jaenisch, R. & Bird, A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genet. 33 (suppl.), 245–254 (2003)
Cheung, P., Allis, C. D. & Sassone-Corsi, P. Signaling to chromatin through histone modifications. Cell 103, 263–271 (2000)
Fischle, W., Wang, Y. & Allis, C. D. Binary switches and modification cassettes in histone biology and beyond. Nature 425, 475–479 (2003)
Felsenfeld, G. & Groudine, M. Controlling the double helix. Nature 421, 448–453 (2003)
Sims, R. J. III, Nishioka, K. & Reinberg, D. Histone lysine methylation: a signature for chromatin function. Trends Genet. 19, 629–639 (2003)
Bannister, A. J., Schneider, R. & Kouzarides, T. Histone methylation: dynamic or static? Cell 109, 801–806 (2002)
Holliday, R. DNA methylation and epigenetic mechanisms. Cell Biophys. 15, 15–20 (1989)
Sassone-Corsi, P. Unique chromatin remodeling and transcriptional regulation in spermatogenesis. Science 296, 2176–2178 (2002)
DeBaun, M. R., Niemitz, E. L. & Feinberg, A. P. Association of in vitro fertilization with Beckwith–Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am . J. Hum. Genet. 72, 156–160 (2003)
Rhind, S. M. et al. Human cloning: can it be made safe? Nature Rev. Genet. 4, 855–864 (2003)
Sarma, K. & Reinberg, D. Histone variants meet their match. Nature Rev. Mol. Cell Biol. 6, 139–149 (2005)
Marston, A. L. & Amon, A. Meiosis: cell-cycle controls shuffle and deal. Nature Rev. Mol. Cell Biol. 5, 983–997 (2004)
Meistrich, M. L., Mohapatra, B., Shirley, C. R. & Zhao, M. Roles of transition nuclear proteins in spermiogenesis. Chromosoma 111, 483–488 (2003)
Yu, Y. E. et al. Abnormal spermatogenesis and reduced fertility in transition nuclear protein 1-deficient mice. Proc. Natl Acad. Sci. USA 97, 4683–4688 (2000)
Zhao, M. et al. Targeted disruption of the transition protein 2 gene affects sperm chromatin structure and reduces fertility in mice. Mol. Cell. Biol. 21, 7243–7255 (2001)
Meetei, A. R., Ullas, K. S., Vasupradha, V. & Rao, M. R. Involvement of protein kinase A in the phosphorylation of spermatidal protein TP2 and its effect on DNA condensation. Biochemistry 41, 185–195 (2002)
Sung, M. T. & Dixon, G. H. Modification of histones during spermiogenesis in trout: a molecular mechanism for altering histone binding to DNA. Proc. Natl Acad. Sci. USA 67, 1616–1623 (1970)
Lahn, B. T. et al. Previously uncharacterized histone acetyltransferases implicated in mammalian spermatogenesis. Proc. Natl Acad. Sci. USA 99, 8707–8712 (2002)
Oliva, R. & Dixon, G. H. Vertebrate protamine genes and the histone-to-protamine replacement reaction. Progr. Nucleic Acid Res. Mol. Biol. 40, 25–94 (1991)
Lewis, J. D. et al. Histone H1 and the origin of protamines. Proc. Natl Acad. Sci. USA 101, 4148–4152 (2004)
Pogany, G. C., Corzett, M., Weston, S. & Balhorn, R. DNA and protein content of mouse sperm. Implications regarding sperm chromatin structure. Exp. Cell Res. 136, 127–136 (1981)
Cho, C. et al. Haploinsufficiency of protamine-1 or -2 causes infertility in mice. Nature Genet. 28, 82–86 (2001)
Wu, J. Y. et al. Spermiogenesis and exchange of basic nuclear proteins are impaired in male germ cells lacking Camk4. Nature Genet. 25, 448–452 (2000)
Xu, X., Toselli, P. A., Russell, L. D. & Seldin, D. C. Globozoospermia in mice lacking the casein kinase IIα′ catalytic subunit. Nature Genet. 23, 118–121 (1999)
Lee, K., Haugen, H. S., Clegg, C. H. & Braun, R. E. Premature translation of protamine 1 mRNA causes precocious nuclear condensation and arrests spermatid differentiation in mice. Proc. Natl Acad. Sci. USA 92, 12451–12455 (1995)
Grimes, S. et al. A rat histone H4 gene closely associated with the testis-specific H1t gene. Exp. Cell Res. 173, 534–545 (1987)
Redon, C. et al. Histone H2A variants H2AX and H2AZ. Curr. Opin. Genet. Dev. 12, 162–169 (2002)
Pandey, N. B. & Marzluff, W. F. The stem-loop structure at the 3′ end of histone mRNA is necessary and sufficient for regulation of histone mRNA stability. Mol. Cell. Biol. 7, 4557–4559 (1987)
Moss, S. B., Challoner, P. B. & Groudine, M. Expression of a novel histone 2B during mouse spermiogenesis. Dev. Biol. 133, 83–92 (1989)
Albig, W. et al. All known human H1 histone genes except the H1(0) gene are clustered on chromosome 6. Genomics 16, 649–654 (1993)
Kobor, M. S. et al. Protein complex containing the conserved Swi2/Snf2-related ATPase Swr1p deposits histone variant H2A.Z into euchromatin. PLoS Biol. 2, E131 (2004)
Tagami, H., Ray-Gallet, D., Almouzni, G. & Nakatani, Y. Histone H3.1 and H3.3 complexes mediat nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116, 51–61 (2004)
Bramlage, B., Kosciessa, U. & Doenecke, D. Differential expression of the murine histone genes H3.3A and H3.3B. Differentiation 62, 13–20 (1997)
Zalensky, A. O. et al. Human testis/sperm-specific histone H2B (hTSH2B). Molecular cloning and characterization. J. Biol. Chem. 277, 43474–43480 (2002)
Martianov, I. et al. Polar nuclear localization of H1T2, a histone H1 variant, required for spermatid elongation and DNA condensation during spermiogenesis. Proc. Natl Acad. Sci. USA 102, 2808–2813 (2005)
Henikoff, S., Ahmad, K., Platero, J. S. & van Steensel, B. Heterochromatic deposition of centromeric histone H3-like proteins. Proc. Natl Acad. Sci. USA 97, 716–721 (2000)
McKittrick, E., Gafken, P. R., Ahmad, K. & Henikoff, S. Histone H3.3 is enriched in covalent modifications associated with active chromatin. Proc. Natl Acad. Sci. USA 101, 1525–1530 (2004)
Crosio, C. et al. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol. Cell. Biol. 22, 874–885 (2002)
Zeitlin, S. G., Shelby, R. D. & Sullivan, K. F. CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J. Cell Biol. 155, 1147–1157 (2001)
Zalensky, A. O. et al. Human testis/sperm-specific histone H2B (hTSH2B). Molecular cloning and characterization. J. Biol. Chem. 277, 43474–43480 (2002)
Niino, Y. S. et al. PKCφ II, a new isoform of protein kinase C specifically expressed in the seminiferous tubules of mouse testis. J. Biol. Chem. 276, 36711–36717 (2001)
Tseng, T. C., Chen, S. H., Hsu, Y. P. & Tang, T. K. Protein kinase profile of sperm and eggs: cloning and characterization of two novel testis-specific protein kinases (AIE1, AIE2) related to yeast and fly chromosome segregation regulators. DNA Cell Biol. 17, 823–833 (1998)
Park, E. J., Chan, D. W., Park, J. H., Oettinger, M. A. & Kwon, J. DNA-PK is activated by nucleosomes and phosphorylates H2AX within the nucleosomes in an acetylation-dependent manner. Nucleic Acids Res. 31, 6819–6827 (2003)
Burma, S., Chen, B. P., Murphy, M., Kurimasa, A. & Chen, D. J. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276, 42462–42467 (2001)
Shroff, R. et al. Distribution and dynamics of chromatin modification induced by a defined DNA double-strand break. Curr. Biol. 14, 1703–1711 (2004)
Celeste, A. et al. Genomic instability in mice lacking histone H2AX. Science 296, 922–927 (2002)
Barlow, C. et al. Atm deficiency results in severe meiotic disruption as early as leptonema of prophase I. Development 125, 4007–4017 (1998)
Abraham, R. T. Checkpoint signaling: epigenetic events sound the DNA strand-breaks alarm to the ATM protein kinase. Bioessays 25, 627–630 (2003)
McKee, B. D. & Handel, M. A. Sex chromosomes, recombination, and chromatin conformation. Chromosoma 102, 71–80 (1993)
Chadwick, B. P. & Willard, H. F. Histone H2A variants and the inactive X chromosome: identification of a second macroH2A variant. Hum. Mol. Genet. 10, 1101–1113 (2001)
Baarends, W. M., Roest, H. P. & Grootegoed, J. A. The ubiquitin system in gametogenesis. Mol. Cell. Endocrinol. 151, 5–16 (1999)
Hendzel, M. J., Lever, M. A., Crawford, E. & Th'ng, J. P. The C-terminal domain is the primary determinant of histone H1 binding to chromatin in vivo . J. Biol. Chem. 279, 20028–20034 (2004)
Fantz, D. A. et al. Mice with a targeted disruption of the H1t gene are fertile and undergo normal changes in structural chromosomal proteins during spermiogenesis. Biol. Reprod. 64, 425–431 (2001)
Doenecke, D. et al. Histone gene expression and chromatin structure during spermatogenesis. Adv. Exp. Med. Biol. 424, 37–48 (1997)
Nayernia, K. et al. Male mice lacking three germ cell expressed genes are fertile. Biol. Reprod. 69, 1973–1978 (2003)
Yan, W., Ma, L., Burns, K. H. & Matzuk, M. M. HILS1 is a spermatid-specific linker histone H1-like protein implicated in chromatin remodeling during mammalian spermiogenesis. Proc. Natl Acad. Sci. USA 100, 10546–10551 (2003)
Contreras, A. et al. The dynamic mobility of histone H1 is regulated by cyclin/CDK phosphorylation. Mol. Cell. Biol. 23, 8626–8636 (2003)
Kuzmichev, A., Jenuwein, T., Tempst, P. & Reinberg, D. Different EZH2-containing complexes target methylation of histone H1 or nucleosomal histone H3. Mol. Cell 14, 183–193 (2004)
Kim, J. M., Liu, H., Tazaki, M., Nagata, M. & Aoki, F. Changes in histone acetylation during mouse oocyte meiosis. J. Cell Biol. 7, 37–46 (2003)
De La Fuente, R., Viveiros, M. M., Wigglesworth, K. & Eppig, J. J. ATRX, a member of the SNF2 family of helicase/ATPases, is required for chromosome alignment and meiotic spindle organization in metaphase II stage mouse oocytes. Dev. Biol. 272, 1–14 (2004)
Jedrusik, M. A. & Schulze, E. A single histone H1 isoform (H1.1) is essential for chromatin silencing and germline development in Caenorhabditis elegans . Development 128, 1069–1080 (2001)
Clarke, H. J., Bustin, M. & Oblin, C. Chromatin modifications during oogenesis in the mouse: removal of somatic subtypes of histone H1 from oocyte chromatin occurs post-natally through a post-transcriptional mechanism. J. Cell Sci. 110, 477–487 (1997)
Debey, P. et al. Competent mouse oocytes isolated from antral follicles exhibit different chromatin organization and follow different maturation dynamics. Mol. Reprod. Dev. 36, 59–74 (1993)
Teranishi, T. et al. Rapid replacement of somatic linker histones with the oocyte-specific linker histone H1Foo in nuclear transfer. Dev. Biol. 266, 76–86 (2004)
Buaas, F. W. et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nature Genet. 36, 647–652 (2004)
Costoya, J. A. et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nature Genet. 36, 653–659 (2004)
Peters, A. H. et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107, 323–337 (2001)
Sedgwick, B. Repairing DNA-methylation damage. Nature Rev. Mol. Cell Biol. 5, 148–157 (2004)
Roest, H. P. et al. Inactivation of the HR6B ubiquitin-conjugating DNA repair enzyme in mice causes male sterility associated with chromatin modification. Cell 86, 799–810 (1996)
Verdel, A. et al. RNAi-mediated targeting of heterochromatin by the RITS complex. Science 303, 672–676 (2004)
Hay, B., Ackerman, L., Barbel, S., Jan, L. Y. & Jan, Y. N. Identification of a component of Drosophila polar granules. Development 103, 625–640 (1988)
Toyooka, Y. et al. Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech. Dev. 93, 139–149 (2000)
Bannister, A. J. et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410, 120–124 (2001)
Pivot-Pajot, C. et al. Acetylation-dependent chromatin reorganization by BRDT, a testis-specific bromodomain-containing protein. Mol. Cell. Biol. 23, 5354–5365 (2003)
Hazzouri, M. et al. Regulated hyperacetylation of core histones during mouse spermatogenesis: involvement of histone deacetylases. Eur. J. Cell Biol. 79, 950–960 (2000)
Brinster, R. L. Germline stem cell transplantation and transgenesis. Science 296, 2174–2176 (2002)
Waterland, R. A. & Jirtle, R. L. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition 20, 63–68 (2004)
Moss, S. B. & Orth, J. M. Localization of a spermatid-specific histone 2B protein in mouse spermiogenic cells. Biol. Reprod. 48, 1047–1056 (1993)
Acknowledgements
We were unfortunately unable to include all the relevant references owing to space constraints. We are grateful to C. D. Allis, D. Reinberg, R. Jaenisch, E. Borrelli, I. Davidson, N. Kotaja, U. Kolthur, G. Fienga, K. Hogeveen, C. Krausz, M. Parvinen, S. Henikoff, R. L. Brinster and all members of the Sassone-Corsi laboratory for critical reading of the manuscript, advice and stimulating discussions. S.K. is supported by fellowships from the Fondation pour la Recherche Médicale and the Marie Curie Programme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Kimmins, S., Sassone-Corsi, P. Chromatin remodelling and epigenetic features of germ cells. Nature 434, 583–589 (2005). https://doi.org/10.1038/nature03368
Issue Date:
DOI: https://doi.org/10.1038/nature03368
This article is cited by
-
Small RNAs, spermatogenesis, and male infertility: a decade of retrospect
Reproductive Biology and Endocrinology (2023)
-
Single-cell RNA-seq uncovers dynamic processes orchestrated by RNA-binding protein DDX43 in chromatin remodeling during spermiogenesis
Nature Communications (2023)
-
Regulation, functions and transmission of bivalent chromatin during mammalian development
Nature Reviews Molecular Cell Biology (2023)
-
Emerging evidence that the mammalian sperm epigenome serves as a template for embryo development
Nature Communications (2023)
-
A reappraisal of the form – function problem. Theory and phenomenology
Theory in Biosciences (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.