Abstract
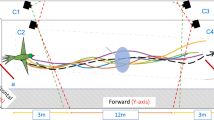
Numerous non-flying arboreal vertebrates use controlled descent (either parachuting or gliding sensu stricto1,2) to avoid predation or to locate resources3,4,5,6,7, and directional control during a jump or fall is thought to be an important stage in the evolution of flight3,8,9. Here we show that workers of the neotropical ant Cephalotes atratus L. (Hymenoptera: Formicidae) use directed aerial descent to return to their home tree trunk with >80% success during a fall. Videotaped falls reveal that C. atratus workers descend abdomen-first through steep glide trajectories at relatively high velocities; a field experiment shows that falling ants use visual cues to locate tree trunks before they hit the forest floor. Smaller workers of C. atratus, and smaller species of Cephalotes more generally, regain contact with their associated tree trunk over shorter vertical distances than do larger workers. Surveys of common arboreal ants suggest that directed descent occurs in most species of the tribe Cephalotini and arboreal Pseudomyrmecinae, but not in arboreal ponerimorphs or Dolichoderinae. This is the first study to document the mechanics and ecological relevance of this form of locomotion in the Earth's most diverse lineage, the insects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Oliver, J. A. “Gliding” in amphibians and reptiles, with a remark on arboreal adaptation in the lizard, Anolis carolinensis carolinensis Voigt. Am. Nat. 85, 171–176 (1951)
Moffett, M. W. What's “up”? A critical look at the basic terms of canopy biology. Biotropica 32, 569–596 (2000)
Kingsolver, J. G. & Koehl, M. A. R. Selective factors in the evolution of insect wings. Annu. Rev. Entomol. 39, 425–451 (1994)
Mori, A. & Hikida, T. Field observations on the social behavior of the flying lizard, Draco volans sumatranus, in Borneo. Copeia 1994, 124–130 (1994)
Jackson, S. M. Glide angle in the genus Petaurus and a review of gliding in mammals. Mamm. Rev. 30, 9–30 (1999)
McCay, M. G. Aerodynamic stability and maneuverability of the gliding frog Polypedates dennysi . J. Exp. Biol. 204, 2817–2826 (2001)
Socha, J. J. Gliding flight in the paradise tree snake. Nature 418, 603–604 (2002)
Maynard Smith, J. The importance of the nervous system in the evolution of animal flight. Evolution 6, 127–129 (1952)
Dudley, R. The Biomechanics of Insect Flight: Form, Function, Evolution 275–287 (Princeton Univ. Press, Princeton, 2000)
Stork, N. E., Adis, J. & Didham, R. K. Canopy Arthropods (Chapman & Hall, London, 1997)
Orivel, J., Malherbe, M. C. & Dejean, A. Relationships between pretarsus morphology and arboreal life in ponerine ants of the genus Pachycondyla (Formicidae: Ponerinae). Ann. Entomol. Soc. Am. 94, 449–456 (2001)
Federle, W., Riehle, M., Curtis, A. S. G. & Full, R. J. An integrative study of insect adhesion: mechanics and wet adhesion of pretarsal pads in ants. Integr. Comp. Biol. 42, 1100–1106 (2002)
Haemig, P. D. Effects of birds on the intensity of ant rain: a terrestrial form of invertebrate drift. Anim. Behav. 54, 89–97 (1997)
Longino, J. T. & Colwell, R. K. Biodiversity assessment using structured inventory: capturing the ant fauna of a tropical rain forest. Ecol. Appl. 7, 1263–1277 (1997)
Weber, N. A. The nest of an anomalous colony of the arboreal ant Cephalotes atratus . Psyche (Cambridge) 64, 60–69 (1957)
Yanoviak, S. P. & Kaspari, M. E. Community structure and the habitat templet: ant assemblages in the tropical canopy and litter. Oikos 89, 259–266 (2000)
Wohlgemuth, S., Ronacher, B. & Wehner, R. Ant odometry in the third dimension. Nature 411, 795–798 (2001)
Hölldobler, B. Canopy orientation: a new kind of orientation in ants. Science 210, 86–88 (1980)
Ellington, C. P. Aerodynamics and the origin of insect flight. Adv. Insect Physiol. 23, 171–210 (1991)
Kempf, W. W. A taxonomic study on the ant tribe Cephalotini (Hymenoptera: Formicidae). Rev. Entomol. 22, 1–244 (1951)
de Andrade, M. L. & Baroni Urbani, C. Diversity and adaptation in the ant genus Cephalotes, past and present. Stuttgarter Beitr. Naturkunde B (Geologie und Paläontologie) 271, 1–889 (1999)
Coyle, F. A. Defensive behavior and associated morphological features in three species of the ant genus Paracryptocerus . Insectes Soc. 13, 93–104 (1966)
Saint-Paul, U. et al. Fish communities in central Amazonian white- and blackwater floodplains. Environ. Biol. Fishes 57, 235–250 (2000)
Wilson, E. O. Behavior of Daceton armigerum (Latreille), with a classification of self-grooming movements in ants. Bull. Mus. Comp. Zool. Harv. 127, 401–422 (1962)
Walker, J. A. Estimating velocities and accelerations of animal locomotion: a simulation experiment comparing numerical differentiation algorithms. J. Exp. Biol. 201, 981–995 (1998)
Acknowledgements
We thank B. Fisher, B. Hölldobler, J. T. Longino, S. Combes, G. Byrnes, W. Lamar, J. B. Gonzáles and C. Saux for helpful discussions, logistical support, and/or comments on the manuscript. J. T. Longino, W. P. Mackay and P. S. Ward assisted with ant identifications. The Smithsonian Tropical Research Institute, the Panamanian National Authority for the Environment (ANAM), and the Peruvian National Institute of Natural Resources (INRENA) provided permits. This project was supported in part by NSF grants to M.K. and an NIH grant to S. C. Weaver (UTMB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Video 1
This video shows an individual worker C. atratus dropped from 30 m height and 3.3 m horizontal distance from the trunk in tree 25 (Supplementary Table 2). The abdomen and hind legs of the ant were painted white, showing the rapid alignment with the trunk and backward glide. (MPG 1776 kb)
Supplementary Video 2
This video shows an individual worker C. atratus painted white and dropped from 25 m height and 2.5 m horizontal distance from the trunk in tree 24 (Supplementary Table 2). The pink flag hanging next to the trunk marks 9.0 m vertical drop distance. (MPG 1275 kb)
Supplementary Video 3
This video shows an individual worker C. atratus painted white and dropped from 25 m height and 2.0 m horizontal distance from the trunk in tree 18 (Supplementary Table 2). (MPG 1656 kb)
Supplementary Methods
This file provides additional background, methods and statistical results related to field experiments. (DOC 35 kb)
Supplementary Figure 1
This figure supports the Supplementary Methods and shows the relationship used to estimate C. atratus worker mass from head width in the field. (DOC 32 kb)
Supplementary Table 1
This table lists the arboreal ant taxa that have been tested for directed aerial descent behaviour. (DOC 33 kb)
Supplementary Table 2
This table provides basic information about the trees used in the study. (DOC 71 kb)
Rights and permissions
About this article
Cite this article
Yanoviak, S., Dudley, R. & Kaspari, M. Directed aerial descent in canopy ants. Nature 433, 624–626 (2005). https://doi.org/10.1038/nature03254
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03254
This article is cited by
-
Multi-modal locomotor costs favor smaller males in a sexually dimorphic leaf-mimicking insect
BMC Ecology and Evolution (2022)
-
How wingless salamanders fly
Nature (2022)
-
Experimental investigations on the strategies of fighting crickets Velarifictorus micado to manipulate air resistance
Science China Physics, Mechanics & Astronomy (2020)
-
Host plant architecture affects the costs of dropping behaviour in Phaedon brassicae (Coleoptera: Chrysomelidae)
Applied Entomology and Zoology (2018)
-
Evidence for contrasting size-frequency distributions of workers patrolling vegetation vs. the ground in the polymorphic African ant Anoplolepis custodiens
Insectes Sociaux (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.