Abstract
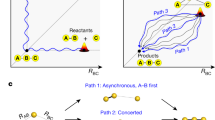
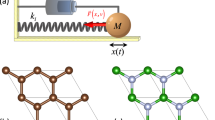
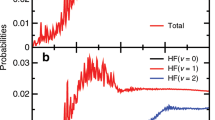
Gaining insight into the nature and dynamics of the transition state is the essence of mechanistic investigations of chemical reactions1, yet the fleeting configuration when existing chemical bonds dissociate while new ones form is extremely difficult to examine directly2. Adiabatic potential-energy surfaces—usually derived using quantum chemical methods3 that assume mutually independent nuclear and electronic motion4—quantify the fundamental forces between atoms involved in reaction and thus provide accurate descriptions of a reacting system as it moves through its transition state5,6. This approach, widely tested for gas-phase reactions7, is now also commonly applied to chemical reactions at metal surfaces8. There is, however, some evidence calling into question the correctness of this theoretical approach for surface reactions: electronic excitation upon highly exothermic chemisorption has been observed9, and indirect evidence suggests that large-amplitude vibrations of reactant molecules can excite electrons at metal surfaces10,11. Here we report the detection of ‘hot’ electrons leaving a metal surface as vibrationally highly excited NO molecules collide with it. Electron emission only occurs once the vibrational energy exceeds the surface work function, and is at least 10,000 times more efficient than the emissions seen in similar systems where large-amplitude vibrations were not involved12,13,14,15,16,17,18. These observations unambiguously demonstrate the direct conversion of vibrational to electronic excitation, thus questioning one of the basic assumptions currently used in theoretical approaches to describing bond-dissociation at metal surfaces.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 3, 107 (1935)
Manolopoulos, D. E. et al. The transition-state of the F + H2 reaction. Science 262, 1852–1855 (1993)
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, 1133–1138 (1965)
Born, M. & Oppenheimer, E. Born Oppenheimer approximation. Ann. Phys. 84, 457–484 (1927)
Eyring, H. & Polanyi, M. Über einfache Gasreaktionen. Sonderdruck aus Z. Phys. Chem. (special issue) B12, 279–311 (1931); Hettema, H. (trans.) in Quantum Chemistry Classic Scientific Papers (World Scientific Series in 20th Century Chemistry Vol. 8, Imperial College Press, River Edge, NJ, 2000).
Eyring, H., Gershinowitz, H. & Sun, C. E. H + H2 potential surface. J. Chem. Phys. 3, 786 (1935)
Chao, S. D. et al. A fully state- and angle-resolved study of the H + HD → D + H2 reaction: Comparison of a molecular beam experiment to ab initio quantum reaction dynamics. J. Chem. Phys. 117, 8341–8361 (2002)
Kroes, G. J., Gross, A., Baerends, E. J., Scheffler, M. & McCormack, D. A. Quantum theory of dissociative chemisorption on metal surfaces. Acc. Chem. Res. 35, 193–200 (2002)
Nienhaus, H. Electronic excitations by chemical reactions on metal surfaces. Surf. Sci. Rep. 45, 3–78 (2002)
Huang, Y. H., Rettner, C. T., Auerbach, D. J. & Wodtke, A. M. Vibrational promotion of electron transfer. Science 290, 111–114 (2000)
Diekhoner, L. et al. Indirect evidence for strong nonadiabatic coupling in N2 associative desorption from and dissociative adsorption on Ru(0001). J. Chem. Phys. 117, 5018–5030 (2002)
Greber, T. Chemical hole diving. Chem. Phys. Lett. 222, 292–296 (1994)
Brandt, M., Greber, T., Bowering, N. & Heinzmann, U. The role of molecular state and orientation in harpooning reactions: N2O on Cs/Pt(111). Phys. Rev. Lett. 81, 2376–2379 (1998)
Brandt, M., Kuhlmann, F., Greber, T., Bowering, N. & Heinzmann, U. Interaction of gas-phase oriented N2O with lithium metal: evidence for an Eley-Rideal mechanism. Surf. Sci. 439, 49–58 (1999)
Bottcher, A. & Giessel, T. Dissociative chemisorption of N2O molecules on Cs layers monitored via exoelectron emission. Surf. Sci. 408, 212–222 (1998)
Bottcher, A., Grobecker, R., Greber, T. & Ertl, G. Negative particle-emission from a Cs/Ru(0001) surface during exposure to NO and NO2 . Chem. Phys. Lett. 208, 404–408 (1993)
Bottcher, A., Grobecker, R., Imbeck, R., Morgante, A. & Ertl, G. Exoelectron emission during oxidation of Cs films. J. Chem. Phys. 95, 3756–3766 (1991)
Grobecker, R., Greber, T., Bottcher, A. & Ertl, G. Thermally activated emission of exoelectrons accompanying the oxidation of Cs films. Phys. Status Solidi A Appl. Res. 146, 259–267 (1994)
Chen, J., Matsiev, D., White, J. D., Murphy, M. & Wodtke, A. M. Hexapole transport and focusing of vibrationally excited NO molecules prepared by optical pumping. Chem. Phys. 301, 161–172 (2004)
Silva, M., Jongma, R., Field, R. W. & Wodtke, A. M. The dynamics of “stretched molecules”: Experimental studies of highly vibrationally excited molecules with stimulated emission pumping. Annu. Rev. Phys. Chem. 52, 811–852 (2001)
Skottkeklein, M. et al. Preparation and characterization of thin CsAu films. Thin Solid Films 203, 131–145 (1991)
Wang, C. S. High photoemission efficiency of submonolayer cesium-covered surfaces. J. Appl. Phys. 48, 1477–1479 (1977)
Woratschek, B., Sesselmann, W., Kuppers, J., Ertl, G. & Haberland, H. The interaction of cesium with oxygen. J. Chem. Phys. 86, 2411–2422 (1987)
Acknowledgements
We thank J. Brauman for reviewing an early version of this manuscript. The work was partially supported by a grant from the National Science Foundation as well as a grant from the Department of Energy Office of Basic Energy Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
White, J., Chen, J., Matsiev, D. et al. Conversion of large-amplitude vibration to electron excitation at a metal surface. Nature 433, 503–505 (2005). https://doi.org/10.1038/nature03213
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03213
This article is cited by
-
Two distinctive energy migration pathways of monolayer molecules on metal nanoparticle surfaces
Nature Communications (2016)
-
Generation of ultra-short hydrogen atom pulses by bunch-compression photolysis
Nature Communications (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.