Abstract
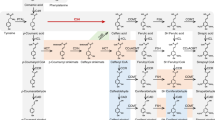
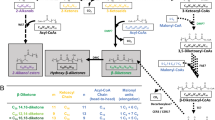
Increasing the l-ascorbate (vitamin C) content of crops could in principle involve promoting its biosynthesis or inhibiting its degradation. Recent progress has revealed biosynthetic pathways for ascorbate1,2,3, but the degradative pathways remain unclear. The elucidation of such pathways could promote an understanding of the roles of ascorbate in plants4, and especially of the intriguing positive correlation between growth rate and ascorbate oxidase5,6 (or its products7). In some plants (Vitaceae), ascorbate is degraded via l-idonate to l-threarate (l-tartrate), with the latter arising from carbons 1–4 of ascorbate3,8,9,10,11. In most plants, however (including Vitaceae)11, ascorbate degradation can occur via dehydroascorbate, yielding oxalate12 plus l-threonate, with the latter from carbons 3–6 of ascorbate3,10,13. The metabolic steps between ascorbate and oxalate/l-threonate, and their subcellular location, were unknown. Here we show that this pathway operates extracellularly in cultured Rosa cells, proceeds via several novel intermediates including 4-O-oxalyl-l-threonate, and involves at least one new enzyme activity. The pathway can also operate non-enzymatically, potentially accounting for vitamin losses during cooking. Several steps in the pathway may generate peroxide; this may contribute to the role of ascorbate as a pro-oxidant14,15 that is potentially capable of loosening the plant cell wall and/or triggering an oxidative burst.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wheeler, G. L., Jones, M. A. & Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 393, 365–369 (1998)
Agius, F. et al. Engineering increased vitamin C levels in plants by overexpression of a d-galacturonic acid reductase. Nature Biotechnol. 21, 177–181 (2003)
Asard, H., May, J. M. & Smirnoff, N. (eds) Vitamin C Function and Biochemistry in Animals and Plants (Bios Scientific Publishers, London, 2004)
Smirnoff, N. The function and metabolism of ascorbic acid in plants. Ann. Bot. 78, 661–669 (1996)
Lin, L. S. & Varner, J. E. Expression of ascorbic acid oxidase in zucchini squash (Cucurbita pepo L). Plant Physiol. 96, 159–165 (1991)
Pignocchi, C., Fletcher, J. M., Wilkinson, J. E., Barnes, J. D. & Foyer, C. H. The function of ascorbate oxidase in tobacco. Plant Physiol. 132, 1631–1641 (2003)
Hidalgo, A., García-Herdugo, G., González-Reyes, J. A., Morré, D. J. & Navas, P. Ascorbate free-radical stimulates onion root growth by increasing cell elongation. Bot. Gaz. 152, 282–288 (1991)
Williams, M. & Loewus, F. A. Biosynthesis of (+ )-tartaric acid from l-ascorbic-4-C14 acid in grape and geranium. Plant Physiol. 61, 672–674 (1978)
Saito, K. & Kasai, Z. Synthesis of l-(+ )-tartaric acid from l-ascorbic-acid via 5-keto-d-gluconic acid in grapes. Plant Physiol. 76, 170–174 (1984)
Saito, K., Ohmoto, J. & Kuriha, N. Incorporation of 18O into oxalic, l-threonic and l-tartaric acids during cleavage of l-ascorbic and 5-keto-d-gluconic acids in plants. Phytochemistry 44, 805–809 (1997)
deBolt, S., Hardie, J., Tyerman, S. & Ford, C. M. Composition and synthesis of raphide crystals and druse crystals in berries of Vitis vinifera L. cv. Cabernet Sauvignon: ascorbic acid as precursor for both oxalic and tartaric acids as revealed by radiolabelling studies. Aust. J. Grape Wine Res. 10, 134–142 (2004)
Yang, J. C. & Loewus, F. A. Metabolic conversion of l-ascorbic-acid to oxalic-acid in oxalate-accumulating plants. Plant Physiol. 56, 283–285 (1975)
Helsper, J. P. & Loewus, F. A. Metabolism of l-threonic acid in Rumex × acutus L. and Pelargonium crispum (L.) l'Hér. Plant Physiol. 69, 1365–1368 (1982)
Podmore, I. D. et al. Vitamin C exhibits pro-oxidant properties. Nature 392, 559 (1998)
Fry, S. C. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem. J. 332, 507–515 (1998)
Takahama, U. Redox state of ascorbic acid in the apoplast of stems of Kalanchoë daigremontiana . Plant Physiol. 89, 791–798 (1993)
Offord, R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature 211, 591–593 (1966)
Deutsch, J. C. Oxygen-accepting antioxidants which arise during ascorbate oxidation. Anal. Biochem. 265, 238–245 (1998)
Dekker, A. O. & Dickinson, R. G. Oxidation of ascorbic acid by oxygen with cupric ion as catalyst. J. Am. Chem. Soc. 62, 2165–2171 (1940)
Lane, B. G., Dunwell, J. M., Ray, J. A., Schmitt, M. R. & Cuming, A. C. Germin, a protein marker of early plant development, is an oxalate oxidase. J. Biol. Chem. 268, 12239–12242 (1993)
Saito, K. Metabolism of l-threotetruronic acid by Pelargonium crispum . Phytochemistry 31, 1219–1222 (1992)
Halliwell, B. & Gutteridge, J. M. C. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 186, 1–85 (1990)
Schopfer, P. Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J. 28, 679–688 (2001)
Rodríguez, A. A., Grunberg, K. A. & Taleisnik, E. L. Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiol. 129, 1627–1632 (2002)
Dumville, J. C. & Fry, S. C. Solubilisation of tomato fruit pectins by ascorbate: a possible non-enzymic mechanism of fruit softening. Planta 217, 951–961 (2003)
Plochl, M., Lyons, T., Ollerenshaw, J. & Barnes, J. Simulating ozone detoxification in the leaf apoplast through the direct reaction with ascorbate. Planta 210, 454–467 (2000)
Conklin, P. L., Williams, E. H. & Last, R. L. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc. Natl Acad. Sci. USA 93, 9970–9974 (1996)
Keates, S. E., Tarlyl, N. M., Loewus, F. A. & Franceschi, V. R. l-Ascorbic acid and l-galactose are sources for oxalic acid and calcium oxalate in Pistia stratiotes . Phytochemistry 53, 433–440 (2000)
Fry, S. C. & Street, H. E. Gibberellin-sensitive suspension cultures. Plant Physiol. 65, 472–477 (1980)
Fry, S. C. The Growing Plant Cell Wall: Chemical and Metabolic Analysis Reprint edn< (Blackburn Press, Caldwell, New Jersey, 2000)
Acknowledgements
We thank B. Dudley and J. Miller for technical assistance. M.A.G. thanks the BBSRC for a research studentship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Green, M., Fry, S. Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-l-threonate. Nature 433, 83–87 (2005). https://doi.org/10.1038/nature03172
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature03172
This article is cited by
-
Protective Role of Melatonin and IAA in the Regulation of Resistance of Potato Regenerants to Cold Stress
Potato Research (2023)
-
Toxicity of zero-valent iron nanoparticles to soil organisms and the associated defense mechanisms: a review
Ecotoxicology (2022)
-
Metabolomic profiling of acerola clones according to the ripening stage
Journal of Food Measurement and Characterization (2021)
-
Changes in lignin structure during earlywood and latewood formation in Scots pine stems
Wood Science and Technology (2019)
-
Transcriptome analysis of Medicago lupulina seedlings leaves treated by high calcium provides insights into calcium oxalate formation
Plant and Soil (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.