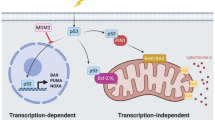
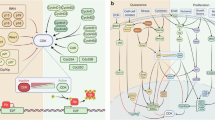
Abstract
Mutations that drive uncontrolled cell-cycle progression are requisite events in tumorigenesis. But evolution has installed in the proliferative programmes of mammalian cells a variety of innate tumour-suppressive mechanisms that trigger apoptosis or senescence, should proliferation become aberrant. These contingent processes rely on a series of sensors and transducers that act in a coordinated network to target the machinery responsible for apoptosis and cell-cycle arrest at different points. Although oncogenic mutations that disable such networks can have profound and varied effects on tumour evolution, they may leave intact latent tumour-suppressive potential that can be harnessed therapeutically.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Evan, G. I. et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell 69, 119–128 (1992).
Evan, G. I. & Vousden, K. H. Proliferation, cell cycle and apoptosis in cancer. Nature 411, 342–348 (2001).
Fridman, J. S. & Lowe, S. W. Control of apoptosis by p53. Oncogene 22, 9030–9040 (2003).
Danial, N. N. & Korsmeyer, S. J. Cell death: critical control points. Cell 116, 205–219 (2004).
Cory, S., Huang, D. C. & Adams, J. M. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22, 8590–8607 (2003).
Green, D. R. & Kroemer, G. The pathophysiology of mitochondrial cell death. Science 305, 626–629 (2004).
Peter, M. E. & Krammer, P. H. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10, 26–35 (2003).
Wilkinson, J. C., Cepero, E., Boise, L. H. & Duckett, C. S. Upstream regulatory role for XIAP in receptor-mediated apoptosis. Mol. Cell. Biol. 24, 7003–7014 (2004).
Scaffidi, C. et al. Two CD95 (APO-1/Fas) signalling pathways. EMBO J. 17, 1675–1687 (1998).
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 408, 307–310 (2000).
Mihara, M. et al. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 11, 577–590 (2003).
White, E. Regulation of the cell cycle and apoptosis by the oncogenes of adenovirus. Oncogene 20, 7836–7846 (2001).
Schmitt, C. A., McCurrach, M. E., de Stanchina, E., Wallace-Brodeur, R. R. & Lowe, S. W. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 13, 2670–2677 (1999).
Symonds, H. et al. p53-dependent apoptosis suppresses tumour growth and progression in vivo. Cell 78, 703–711 (1994).
Pierce, A. M. et al. Increased E2F1 activity induces skin tumours in mice heterozygous and nullizygous for p53. Proc. Natl Acad. Sci. USA 95, 8858–8863 (1998).
Yin, C., Knudson, C. M., Korsmeyer, S. J. & Van Dyke, T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 385, 637–640 (1997).
Hemann, M. T. et al. Suppression of tumorigenesis by the p53 target PUMA. Proc. Natl Acad. Sci. USA 101, 9333–9338 (2004).
Schmitt, C. A. et al. Dissecting p53 tumour suppressor functions in vivo. Cancer Cell 1, 289–298 (2002).
Pelengaris, S., Khan, M. & Evan, G. I. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell 109, 321–334 (2002).
Lowe, S. W. & Sherr, C. J. Tumour suppression by Ink4a-Arf: progress and puzzles. Curr. Opin. Genet. Dev. 13, 77–83 (2003).
Sherr, C. J. The INK4a/ARF network in tumour suppression. Nature Rev. Mol. Cell Biol. 2, 731–737 (2001).
Zindy, F. et al. Myc signalling via the ARF tumour suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 12, 2424–2433 (1998).
de Stanchina, E. et al. E1A signalling to p53 involves the p19(ARF) tumour suppressor. Genes Dev. 12, 2434–2442 (1998).
Kamijo, T. et al. Tumour suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91, 649–659 (1997).
Schmitt, C. A. et al. A senescence programme controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 109, 335–346 (2002).
Jacobs, J. J. et al. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev. 13, 2678–2690 (1999).
Eischen, C. M., Weber, J. D., Roussel, M. F., Sherr, C. J. & Cleveland, J. L. Disruption of the ARF–Mdm2–p53 tumour suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 13, 2658–2669 (1999).
Verschuren, E. W., Klefstrom, J., Evan, G. I. & Jones, N. The oncogenic potential of Kaposi's sarcoma-associated herpesvirus cyclin is exposed by p53 loss in vitro and in vivo. Cancer Cell 2, 229–241 (2002).
Tolbert, D., Lu, X., Yin, C., Tantama, M. & Van Dyke, T. p19(ARF) is dispensable for oncogenic stress-induced p53-mediated apoptosis and tumour suppression in vivo. Mol. Cell. Biol. 22, 370–377 (2002).
Khan, S. H., Moritsugu, J. & Wahl, G. M. Differential requirement for p19ARF in the p53-dependent arrest induced by DNA damage, microtubule disruption, and ribonucleotide depletion. Proc. Natl Acad. Sci. USA 97, 3266–3271 (2000).
Rogoff, H. A. et al. Apoptosis associated with deregulated E2F activity is dependent on E2F1 and Atm/Nbs1/Chk2. Mol. Cell. Biol. 24, 2968–2977 (2004).
Liao, M. J., Yin, C., Barlow, C., Wynshaw-Boris, A. & van Dyke, T. Atm is dispensable for p53 apoptosis and tumour suppression triggered by cell cycle dysfunction. Mol. Cell. Biol. 19, 3095–3102 (1999).
Conn, C. W., Lewellyn, A. L. & Maller, J. L. The DNA damage checkpoint in embryonic cell cycles is dependent on the DNA-to-cytoplasmic ratio. Dev. Cell 7, 275–281 (2004).
Flores, E. R. et al. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 416, 560–564 (2002).
Urist, M. & Prives, C. p53 leans on its siblings. Cancer Cell 1, 311–313 (2002).
Yang, A., Kaghad, M., Caput, D. & McKeon, F. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 18, 90–95 (2002).
Senoo, M., Manis, J. P., Alt, F. W. & McKeon, F. p63 and p73 are not required for the development and p53-dependent apoptosis of T cells. Cancer Cell 6, 85–89 (2004).
Irwin, M. S. et al. Chemosensitivity linked to p73 function. Cancer Cell 3, 403–410 (2003).
Bergamaschi, D. et al. p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell 3, 387–402 (2003).
Gaiddon, C., Lokshin, M., Ahn, J., Zhang, T. & Prives, C. A subset of tumour-derived mutant forms of p53 down-regulate p63 and p73 through a direct interaction with the p53 core domain. Mol. Cell. Biol. 21, 1874–1887 (2001).
Perez, D. & White, E. E1A sensitizes cells to tumour necrosis factor alpha by downregulating c-FLIP S. J. Virol. 77, 2651–2662 (2003).
Klefstrom, J., Verschuren, E. W. & Evan, G. c-Myc augments the apoptotic activity of cytosolic death receptor signalling proteins by engaging the mitochondrial apoptotic pathway. J. Biol. Chem. 277, 43224–43232 (2002).
Croxton, R., Ma, Y., Song, L., Haura, E. B. & Cress, W. D. Direct repression of the Mcl-1 promoter by E2F1. Oncogene 21, 1359–1369 (2002).
Eischen, C. M. et al. Bcl-2 is an apoptotic target suppressed by both c-Myc and E2F-1. Oncogene 20, 6983–6993 (2001).
Egle, A., Harris, A. W., Bouillet, P. & Cory, S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc. Natl Acad. Sci. USA 101, 6164–6169 (2004).
Hershko, T. & Ginsberg, D. Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J. Biol. Chem. 279, 8627–8634 (2004).
Nahle, Z. et al. Direct coupling of the cell cycle and cell death machinery by E2F. Nature Cell Biol. 4, 859–864 (2002).
Sears, R., Ohtani, K. & Nevins, J. R. Identification of positively and negatively acting elements regulating expression of the E2F2 gene in response to cell growth signals. Mol. Cell. Biol. 17, 5227–5235 (1997).
Matsumura, I., Tanaka, H. & Kanakura, Y. E2F1 and c-Myc in cell growth and death. Cell Cycle 2, 333–338 (2003).
Leone, G. et al. Myc requires distinct E2F activities to induce S phase and apoptosis. Mol. Cell 8, 105–113 (2001).
Russell, J. L. et al. ARF differentially modulates apoptosis induced by E2F1 and Myc. Mol. Cell. Biol. 22, 1360–1368 (2002).
Baudino, T. A. et al. Myc-mediated proliferation and lymphomagenesis, but not apoptosis, are compromised by E2f1 loss. Mol. Cell 11, 905–914 (2003).
Conner, E. A. et al. Dual functions of E2F-1 in a transgenic mouse model of liver carcinogenesis. Oncogene 19, 5054–5062 (2000).
Leone, G., DeGregori, J., Sears, R., Jakoi, L. & Nevins, J. R. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature 387, 422–426 (1997).
Dimri, G. P., Itahana, K., Acosta, M. & Campisi, J. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumour suppressor. Mol. Cell. Biol. 20, 273–285 (2000).
Damalas, A., Kahan, S., Shtutman, M., Ben-Ze'ev, A. & Oren, M. Deregulated beta-catenin induces a p53- and ARF-dependent growth arrest and cooperates with Ras in transformation. EMBO J. 20, 4912–4922 (2001).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Campisi, J. Cellular senescence as a tumour-suppressor mechanism. Trends Cell Biol. 11, S27–S31 (2001).
Shay, J. W. & Roninson, I. B. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene 23, 2919–2933 (2004).
Pearson, M. et al. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature 406, 207–210 (2000).
Ferbeyre, G. et al. PML is induced by oncogenic ras and promotes premature senescence. Genes Dev. 14, 2015–2027 (2000).
Itahana, K. et al. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol. Cell. Biol. 23, 389–401 (2003).
Narita, M. et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716 (2003).
Paramio, J. M. et al. The ink4a/arf tumour suppressors cooperate with p21cip1/waf in the processes of mouse epidermal differentiation, senescence, and carcinogenesis. J. Biol. Chem. 276, 44203–44211 (2001).
Elenbaas, B. et al. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 15, 50–65 (2001).
Horner, S. M., DeFilippis, R. A., Manuelidis, L. & DiMaio, D. Repression of the human papillomavirus E6 gene initiates p53-dependent, telomerase-independent senescence and apoptosis in HeLa cervical carcinoma cells. J. Virol. 78, 4063–4073 (2004).
Wu, X. & Levine, A. J. p53 and E2F-1 cooperate to mediate apoptosis. Proc. Natl Acad. Sci. USA 91, 3602–3606 (1994).
Benanti, J. A. & Galloway, D. A. Normal human fibroblasts are resistant to RAS-induced senescence. Mol. Cell. Biol. 24, 2842–2852 (2004).
Tuveson, D. A. et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5, 375–387 (2004).
Guerra, C. et al. Tumour induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell 4, 111–120 (2003).
Lin, A. W. & Lowe, S. W. Oncogenic ras activates the ARF-p53 pathway to suppress epithelial cell transformation. Proc. Natl Acad. Sci. USA 98, 5025–5030 (2001).
Kelly-Spratt, K. S., Gurley, K. E., Yasui, Y. & Kemp, C. J. p19(Arf) suppresses growth, progression, and metastasis of Hras-driven carcinomas through p53-dependent and -independent pathways. PLoS Biol. 2, E242 (2004).
Pelengaris, S., Littlewood, T., Khan, M., Elia, G. & Evan, G. Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol. Cell 3, 565–577 (1999).
Raff, M. C. Social controls on cell survival and cell death. Nature 356, 397–400 (1992).
Askew, D., Ashmun, R., Simmons, B. & Cleveland, J. Constitutive c-myc expression in IL-3-dependent myeloid cell line suppresses cycle arrest and accelerates apoptosis. Oncogene 6, 1915–1922 (1991).
Harrington, E. A., Bennett, M. R., Fanidi, A. & Evan, G. I. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 13, 3286–3295 (1994).
Grossmann, J. Molecular mechanisms of ‘detachment-induced apoptosis–Anoikis’. Apoptosis 7, 247–260 (2002).
Vivanco, I. & Sawyers, C. L. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nature Rev. Cancer 2, 489–501 (2002).
Plas, D. R., Rathmell, J. C. & Thompson, C. B. Homeostatic control of lymphocyte survival: potential origins and implications. Nature Immunol. 3, 515–521 (2002).
Grad, J. M., Zeng, X. R. & Boise, L. H. Regulation of Bcl-xL: a little bit of this and a little bit of STAT. Curr. Opin. Oncol. 12, 543–549 (2000).
LeRoith, D. & Helman, L. The new kid on the block(ade) of the IGF-1 receptor. Cancer Cell 5, 201–202 (2004).
Christofori, G., Naik, P. & Hanahan, D. A second signal supplied by insulin-like growth factor II in oncogene-induced tumorigenesis. Nature 369, 414–418 (1994).
Orsulic, S. et al. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell 1, 53–62 (2002).
Kauffmann-Zeh, A. et al. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature 385, 544–548 (1997).
Wendel, H. G. et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature 428, 332–337 (2004).
Zindy, F. et al. Arf tumour suppressor promoter monitors latent oncogenic signals in vivo. Proc. Natl Acad. Sci. USA 100, 15930–15935 (2003).
Aslanian, A., Iaquinta, P. J., Verona, R. & Lees, J. A. Repression of the Arf tumour suppressor by E2F3 is required for normal cell cycle kinetics. Genes Dev. 18, 1413–1422 (2004).
Jin, S. et al. CIAP1 and the serine protease HTRA2 are involved in a novel p53-dependent apoptosis pathway in mammals. Genes Dev. 17, 359–367 (2003).
Zheng, T. S. et al. Deficiency in caspase-9 or caspase-3 induces compensatory caspase activation. Nature Med. 6, 1241–1247 (2000).
Harlin, H., Reffey, S. B., Duckett, C. S., Lindsten, T. & Thompson, C. B. Characterization of XIAP-deficient mice. Mol. Cell. Biol. 21, 3604–3608 (2001).
Sage, J., Miller, A. L., Perez-Mancera, P. A., Wysocki, J. M. & Jacks, T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature 424, 223–228 (2003).
Seoane, J., Le, H. V. & Massague, J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature 419, 729–734 (2002).
Johnstone, R. W., Ruefli, A. A. & Lowe, S. W. Apoptosis: a link between cancer genetics and chemotherapy. Cell 108, 153–164 (2002).
Chin, L. et al. Essential role for oncogenic Ras in tumour maintenance. Nature 400, 468–472 (1999).
Felsher, D. W. & Bishop, J. M. Reversible tumorigenesis by MYC in haematopoietic lineages. Mol. Cell 4, 199–207 (1999).
Gunther, E. J. et al. Impact of p53 loss on reversal and recurrence of conditional Wnt-induced tumorigenesis. Genes Dev. 17, 488–501 (2003).
Moody, S. E. et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell 2, 451–461 (2002).
Pakunlu, R. I., Cook, T. J. & Minko, T. Simultaneous modulation of multidrug resistance and antiapoptotic cellular defence by MDR1 and BCL-2 targeted antisense oligonucleotides enhances the anticancer efficacy of doxorubicin. Pharm. Res. 20, 351–359 (2003).
Bykov, V. J. & Wiman, K. G. Novel cancer therapy by reactivation of the p53 apoptosis pathway. Ann. Med. 35, 458–465 (2003).
Acknowledgements
We thank members of the Lowe and Evan laboratories for discussions, and M. Hemann, D. Burgess and M. McCurrach for critical readings of the manuscript. S. L. is supported by an NFCR-AACR research professorship and grants from the National Institutes of Health and the Leukemia and Lymphoma Society, E. C. is supported by a postdoctoral training fellowship from the National Cancer Institute, and G. E. is supported by grants from the NIH, the Juvenile Diabetes Foundation and the Brain Tumor Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lowe, S., Cepero, E. & Evan, G. Intrinsic tumour suppression. Nature 432, 307–315 (2004). https://doi.org/10.1038/nature03098
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature03098
This article is cited by
-
50 years on and still very much alive: ‘Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics’
British Journal of Cancer (2023)
-
Sub-lethal doses of chemotherapeutic agents induce senescence in T cells and upregulation of PD-1 expression
Clinical and Experimental Medicine (2023)
-
Enhanced O-GlcNAc modification induced by the RAS/MAPK/CDK1 pathway is required for SOX2 protein expression and generation of cancer stem cells
Scientific Reports (2022)
-
Embelin downregulated cFLIP in breast cancer cell lines facilitate anti-tumor effect of IL-1β-stimulated human umbilical cord mesenchymal stem cells
Scientific Reports (2021)
-
Human cell transformation by combined lineage conversion and oncogene expression
Oncogene (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.