Abstract
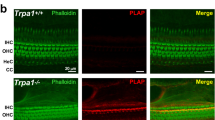
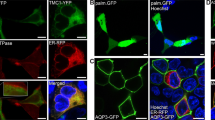
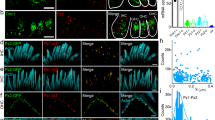
Mechanical deflection of the sensory hair bundles of receptor cells in the inner ear causes ion channels located at the tips of the bundle to open, thereby initiating the perception of sound. Although some protein constituents of the transduction apparatus are known, the mechanically gated transduction channels have not been identified in higher vertebrates. Here, we investigate TRP (transient receptor potential) ion channels as candidates and find one, TRPA1 (also known as ANKTM1), that meets criteria for the transduction channel. The appearance of TRPA1 messenger RNA expression in hair cell epithelia coincides developmentally with the onset of mechanosensitivity. Antibodies to TRPA1 label hair bundles, especially at their tips, and tip labelling disappears when the transduction apparatus is chemically disrupted. Inhibition of TRPA1 protein expression in zebrafish and mouse inner ears inhibits receptor cell function, as assessed with electrical recording and with accumulation of a channel-permeant fluorescent dye. TRPA1 is probably a component of the transduction channel itself.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hudspeth, A. J. & Corey, D. P. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc. Natl Acad. Sci. USA 74, 2407–2411 (1977)
Hudspeth, A. J. Extracellular current flow and the site of transduction by vertebrate hair cells. J. Neurosci. 2, 1–10 (1982)
Corey, D. P. & Hudspeth, A. J. Response latency of vertebrate hair cells. Biophys. J. 26, 499–506 (1979)
Corey, D. P. & Hudspeth, A. J. Kinetics of the receptor current in bullfrog saccular hair cells. J. Neurosci. 3, 962–976 (1983)
Sukharev, S. & Corey, D. P. Mechanosensitive channels: multiplicity of families and gating paradigms. Sci. STKE 2004, re4 (2004)
Corey, D. P. & Hudspeth, A. J. Ionic basis of the receptor potential in a vertebrate hair cell. Nature 281, 675–677 (1979)
Gale, J. E., Marcotti, W., Kennedy, H. J., Kros, C. J. & Richardson, G. P. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J. Neurosci. 21, 7013–7025 (2001)
Meyers, J. R. et al. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J. Neurosci. 23, 4054–4065 (2003)
García-Añoveros, J., García, J. A., Liu, J.-D. & Corey, D. P. The nematode degenerin UNC-105 forms ion channels that are activated by degeneration- or hypercontraction-causing mutations. Neuron 20, 1231–1241 (1998)
Duggan, A., García-Añoveros, J. & Corey, D. P. Insect mechanoreception: What a long, strange TRP it's been. Curr. Biol. 10, R384–R387 (2000)
Clapham, D. E., Montell, C., Schultz, G. & Julius, D. International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: transient receptor potential channels. Pharmacol. Rev. 55, 591–596 (2003)
Clapham, D. E. TRP channels as cellular sensors. Nature 426, 517–524 (2003)
Corey, D. P. New TRP channels in hearing and mechanosensation. Neuron 39, 585–588 (2003)
Walker, R. G., Willingham, A. T. & Zuker, C. S. A Drosophila mechanosensory transduction channel. Science 287, 2229–2234 (2000)
Sidi, S., Friedrich, R. W. & Nicolson, T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science 301, 96–99 (2003)
Story, G. M. et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829 (2003)
Jordt, S. E. et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265 (2004)
Bandell, M. et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857 (2004)
Bermingham, N. A. et al. Math1: an essential gene for the generation of inner ear hair cells. Science 284, 1837–1841 (1999)
Di Palma, F. et al. Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc. Natl Acad. Sci. USA 99, 14994–14999 (2002)
Geleoc, G. S. & Holt, J. R. Developmental acquisition of sensory transduction in hair cells of the mouse inner ear. Nature Neurosci. 6, 1019–1020 (2003)
Denman-Johnson, K. & Forge, A. Establishment of hair bundle polarity and orientation in the developing vestibular system of the mouse. J. Neurocytol. 28, 821–835 (1999)
Hasson, T. et al. Unconventional myosins in inner-ear sensory epithelia. J. Cell Biol. 137, 1287–1307 (1997)
Siemens, J. et al. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature 428, 950–955 (2004)
Reiners, J. et al. Differential distribution of harmonin isoforms and their possible role in Usher-1 protein complexes in mammalian photoreceptor cells. Invest. Ophthalmol. Vis. Sci. 44, 5006–5015 (2003)
Assad, J. A., Shepherd, G. M. & Corey, D. P. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7, 985–994 (1991)
Holt, J. R. et al. Functional expression of exogenous proteins in mammalian sensory hair cells infected with adenoviral vectors. J. Neurophysiol. 81, 1881–1888 (1999)
Hodges, B. L. et al. Multiply deleted [E1, polymerase-, and pTP-] adenovirus vector persists despite deletion of the preterminal protein. J. Gene Med. 2, 250–259 (2000)
Luebke, A. E., Steiger, J. D., Hodges, B. L. & Amalfitano, A. A modified adenovirus can transfect cochlear hair cells in vivo without compromising cochlear function. Gene Ther. 8, 789–794 (2001)
Holt, J. R. Viral-mediated gene transfer to study the molecular physiology of the mammalian inner ear. Audiol. Neurootol. 7, 157–160 (2002)
Farris, H. E., LeBlanc, C. L., Goswami, J. & Ricci, A. J. Probing the pore of the auditory hair cell mechanotransducer channel in turtle. J. Physiol. 558, 769–792 (2004)
Gillespie, P. G. & Corey, D. P. Myosin and adaptation by hair cells. Neuron 19, 955–958 (1997)
Corey, D. P. & Sotomayor, M. Hearing: tightrope act. Nature 428, 901–903 (2004)
Howard, J. & Bechstedt, S. Hypothesis: a helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors. Curr. Biol. 14, R224–R226 (2004)
Howard, J. & Hudspeth, A. J. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog's saccular hair cell. Neuron 1, 189–199 (1988)
Sollner, C. et al. Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature 428, 955–959 (2004)
Michaely, P., Tomchick, D. R., Machius, M. & Anderson, R. G. Crystal structure of a 12 ANK repeat stack from human ankyrinR. EMBO J. 21, 6387–6396 (2002)
Kachar, B., Parakkal, M., Kurc, M., Zhao, Y. & Gillespie, P. G. High-resolution structure of hair-cell tip links. Proc. Natl Acad. Sci. USA 97, 13336–13341 (2000)
Ricci, A. J., Wu, Y. C. & Fettiplace, R. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J. Neurosci. 18, 8261–8277 (1998)
Hudspeth, A. J., Choe, Y., Mehta, A. D. & Martin, P. Putting ion channels to work: mechanoelectrical transduction, adaptation, and amplification by hair cells. Proc. Natl Acad. Sci. USA 97, 11765–11772 (2000)
Starr, C. J., Kappler, J. A., Chan, D. K., Kollmar, R. & Hudspeth, A. J. Mutation of the zebrafish choroideremia gene encoding Rab escort protein 1 devastates hair cells. Proc. Natl Acad. Sci. USA 101, 2572–2577 (2004)
He, T. C. et al. A simplified system for generating recombinant adenoviruses. Proc. Natl Acad. Sci. USA 95, 2509–2514 (1998)
Acknowledgements
Authors' contributions are listed in Supplementary Information. We thank C.-L. Chen and Q. Ma for assistance with in situ hybridization, N. Hopkins for zebrafish support and L. Stevens for laboratory administration. This work was supported by grants from NIH to N. Hopkins, S.-Y.L., J.G.-A., G.S.G.G., J.R.H. and D.P.C.; from the Mathers Foundation to D.P.C.; from the Howard Hughes Medical Institute (J.G.-A.); and from the Charles Dana Foundation to Q. Ma. P.G. was a Parker B. Francis Fellow in Pulmonary Medicine. J.G.-A., A.D., G.G., J.R.H. and H.L.R. were Associates, M.A.V. and K.K. are Associates, and D.P.C. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Information
Contains Supplementary Figures 1–4, as well as supplementary methods and details of author’s contributions. (PDF 862 kb)
Rights and permissions
About this article
Cite this article
Corey, D., García-Añoveros, J., Holt, J. et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432, 723–730 (2004). https://doi.org/10.1038/nature03066
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature03066
This article is cited by
-
Identification of RNA reads encoding different channels in isolated rat ventricular myocytes and the effect of cell stretching on L-type Ca2+current
Biology Direct (2023)
-
TRPA1 activation in non-sensory supporting cells contributes to regulation of cochlear sensitivity after acoustic trauma
Nature Communications (2023)
-
Molecular architecture and gating mechanisms of the Drosophila TRPA1 channel
Cell Discovery (2023)
-
TRPA1s act as chemosensors but not as cold sensors or mechanosensors to trigger the swallowing reflex in rats
Scientific Reports (2022)
-
Gut feelings: mechanosensing in the gastrointestinal tract
Nature Reviews Gastroenterology & Hepatology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.