Abstract
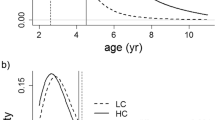
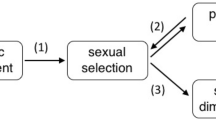
Senescence may result from an optimal balance between current reproductive investment and bodily repair processes required for future reproduction1, a theoretical prediction difficult to prove especially in large, long-lived animals. Here we propose that teeth that have fixed dimensions early in life, but that wear during chewing, can be taken as a measure of total lifetime ‘repair’, and their wear rate as a measure of current expenditure in performance. Our approach also considers the sexual selection process to investigate the advance of senescence in males compared with females, when selection favouring competition over mates reduces the reproductive lifespan of males2. We studied carcasses of 2,141 male and 739 female red deer (Cervus elaphus) of different ages, finding that male molariform teeth emerged at a far smaller size than expected from body size dimorphism. This led to higher workload, steeper wear rate and earlier depletion of male teeth than in females, in concordance with sex-specific patterns of lifetime performance and reproduction. These findings provide the empirical support for the disposable-soma hypothesis of senescence3, which predicts that investment in bodily repair will decrease when the return from this investment may not be realized as a result of other causes that limit survival or reproduction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kirkwood, T. B. L. & Austad, S. N. Why do we age? Nature 408, 233–238 (2000)
Andersson, M. Sexual Selection (Princeton Univ. Press, Princeton, New Jersey, 1994)
Kirkwood, T. B. L. in Handbook of the Biology of Ageing (eds Finch, C. E. & Schneider, E. L.) 27–44 (Van Nostrand Reinhold, New York, 1985)
Medawar, P. B. Old age and natural death. Mod. Q. 1, 30–56 (1946)
Edney, R. B. & Gill, R. W. Evolution of senescence and specific longevity. Nature 220, 281–282 (1968)
Williams, G. C. Pleiotropy, natural selection and the evolution of senescence. Evolution 11, 398–411 (1957)
Hamilton, W. D. The moulding of senescence by natural selection. J. Theor. Biol. 12, 12–45 (1966)
Rose, M. R. Evolutionary Biology of Aging (Oxford Univ. Press, New York, 1991)
Kirkwood, T. B. L. & Rose, M. R. Evolution of senescence: late survival sacrificed for reproduction. Phil. Trans. R. Soc. Lond. B 332, 15–24 (1991)
Haldane, J. B. S. New Paths in Genetics (Allen & Unwin, London, 1941)
Ricklefs, R. E. Evolutionary theories of ageing: confirmation of a fundamental prediction, with implications for the genetic basis and evolution of lifespan. Am. Nat. 152, 24–44 (1998)
Tyler, N. J. C. Natural Limitation of the Abundance of the High Arctic Svalbard Reindeer. Thesis, Univ. Cambridge (1987)
Skogland, T. Tooth wear by food limitation and its life history consequences in wild reindeer. Oikos 51, 238–242 (1988)
Gaillard, J.-M., Delorme, D., Boutin, J.-M., Van Laere, G. & Pradel, R. Roe deer survival patterns: a comparative analysis of contrasting populations. J. Anim. Ecol. 62, 778–791 (1993)
Ericsson, G. & Wallin, K. Age-specific moose (Alces alces) mortality in a predator-free environment: evidence for senescence in females. Ecoscience 8, 157–163 (2001)
Loe, L. E., Mysterud, A., Langvatn, R. & Stenseth, N. C. Decelerating and sex-dependent tooth wear in Norwegian red deer. Oecologia 135, 346–353 (2003)
Clutton-Brock, T. H., Albon, S. D. & Guinness, F. E. in Reproductive Success (ed. Clutton-Brock, T. H.) 325–343 (Chicago Univ. Press, Chicago, 1988)
Gompertz, B. On the nature of the function expressive of the law of human mortality and on the new mode of determining life contingencies. Phil. Trans. R. Soc. Lond. 115, 513–585 (1825)
Clutton-Brock, T. H., Guinness, F. E. & Albon, S. D. Red Deer. Behaviour and Ecology of Two Sexes (Edinburgh Univ. Press, Edinburgh, 1982)
Mysterud, A., Yoccoz, N. G., Stenseth, N. C. & Langvatn, R. Effects of age, sex, and density on body weight of Norwegian red deer: evidence of density-dependence senescence. Proc. R. Soc. Lond. B 268, 911–919 (2001)
Fortelius, M. Ungulate cheek teeth: developmental, functional, and evolutionary interrelations. Acta Zool. Fenn. 180, 1–76 (1985)
Lucas, P. W. Dental Functional Morphology (Cambridge Univ. Press, Cambridge, 2004)
Staines, B. W. & Crisp, J. M. Observations on food quality in Scottish red deer (Cervus elaphus) as determined by chemical analysis of the rumen contents. J. Zool. 185, 253–259 (1978)
Clutton-Brock, T. H., Iason, G. R. & Guinness, F. E. Sexual segregation and density related changes in habitat use in female and male red deer (Cervus elaphus L.). J. Zool. 211, 275–289 (1987)
Mysterud, A. The relationship between ecological segregation and sexual body-size dimorphism in large herbivores. Oecologia 124, 40–54 (2000)
Conradt, L., Gordon, I. J., Clutton-Brock, T. H., Thomson, D. & Guinness, F. E. Could the indirect competition hypothesis explain inter-sexual site segregation in red deer (Cervus elaphus L.)? J. Zool. 254, 285–293 (2001)
Bonenfant, C., Gaillard, J.-M., Loison, A. & Klein, F. Sex-ratio variation and reproductive costs in relation to density in a forest-dwelling population of red deer. Behav. Ecol. 14, 862–869 (2003)
Mitchell, B. Growth layers in dental cement for determining the age of red deer (Cervus elaphus L.). J. Anim. Ecol. 36, 279–293 (1967)
Acknowledgements
We thank T. Kirkwood, P. W. Lucas, A. Mysterud and J. Pérez-Barbería for comments; R. Álvarez, L. Castillo, P. Cidoncha, A. Flores, B. Gutiérrez, J. G. Martínez, Y. Moreno, S. del Río and B. Sánchez for help in field and laboratory work, the Dirección General de Medio Ambiente of Extremadura for permissions and facilities, and J. A. Campón for allowing data and sample collection. Financial support came from Spanish Ministry of Science, FEDER and Junta de Extremadura (Consejería de Agricultura y Medio Ambiente, and Consejería de Educación Ciencia y Tecnología). C.B.S.P. and S.A. were supported by a predoctoral grant of the Autonomic Government of Extremadura.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Table 1
Relationships of dental variables and body size variables with age in males and females. Equations of regression lines were used to obtain maximum trait values in males and females to estimate sexual dimorphism (see Methods). (DOC 23 kb)
Supplementary Figure 1
Variation of the index of workload on occlusal surface areas of postcanine teeth along age for males and females. (DOC 54 kb)
Rights and permissions
About this article
Cite this article
Carranza, J., Alarcos, S., Sánchez-Prieto, C. et al. Disposable-soma senescence mediated by sexual selection in an ungulate. Nature 432, 215–218 (2004). https://doi.org/10.1038/nature03004
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03004
This article is cited by
-
Investigating the domestication and early management of reindeer (Rangifer tarandus) in the Sámi archaeological context from teeth geometric morphometrics
Scientific Reports (2023)
-
The dark-ventral-patch of male red deer, a sexual signal that conveys the degree of involvement in rutting behavior
BMC Zoology (2021)
-
Dentition and body condition: tooth wear as a correlate of weight loss in roe deer
Frontiers in Zoology (2021)
-
Social environment with high intrasexual competition enhances the positive relationship between faecal testosterone and cortisol metabolite levels in red deer
Mammalian Biology (2021)
-
Effects of age on foraging behavior in two closely related albatross species
Movement Ecology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.