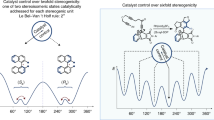
Abstract
Many receptors1 and allosteric proteins2 function through binding of a molecule to induce a conformational change, which then influences a remote active site. In synthetic systems, comparable intramolecular information transfer can be effected by using the shape of one part of a molecule to control the stereoselectivity of reactions occurring some distance away3. However, the need for direct communication with the reaction site usually limits such remote stereocontrol to distances of not more than about five bond lengths. Cyclic structures overcome this problem by allowing the controlling centre and the reaction site4,5 to approach each other, but the information transfer spans only short absolute distances. Truly remote stereocontrol can, however, be achieved with rigid compounds containing amide groups: the conformation of the amides can be controlled by stereogenic centres6,7,8,9 and responds to that of neighbouring amide groups10,11,12 and in turn influences stereoselective reactions13. This strategy has allowed remote stereocontrol spanning 8 (ref. 11) or 9 (ref. 12) bonds. Here we demonstrate stereocontrol over a reaction taking place more than 20 bond lengths from the controlling centre, corresponding to a linear distance of over 2.5 nm. This transmission of information, achieved by conformational changes relayed through the molecule, provides a chemical model of allostery and might serve as a molecular mechanism for communicating and processing information14,15,16.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Krauss, G. Biochemistry of Signal Transduction and Regulation (Wiley-VCH, Weinheim, 1999)
Perutz, M. Mechanisms of Cooperativity and Allosteric Regulation in Proteins (Cambridge Univ. Press, Cambridge, 1990)
Eliel, E. L. & Wilen, S. H. Stereochemistry of Organic Compounds Ch. 12 (Wiley, New York, 1994)
Mikami, K., Shimizu, M., Zhang, H.-C. & Maryanoff, B. E. Acyclic stereocontrol between remote atom centers via intramolecular and intermolecular stereo-communication. Tetrahedron 57, 2917–2951 (2001)
Linnane, P., Magnus, N. & Magnus, P. Induction of molecular asymmetry by a remote chiral group. Nature 385, 799–801 (1997)
Clayden, J., Mitjans, D. & Youssef, L. H. Lithium-sulfoxide-lithium exchange for the asymmetric synthesis of atropisomers under thermodynamic control. J. Am. Chem. Soc. 124, 5266–5267 (2002)
Clayden, J. & Lai, L. W. (- )-Ephedrine as an auxiliary for the asymmetric synthesis of atropisomeric amides by dynamic resolution under thermodynamic control. Tetrahedron Lett. 42, 3163–3166 (2001)
Clayden, J., Lai, L. W. & Helliwell, M. Using amide conformation to “project” the stereochemistry of a (- )-ephedrine-derived oxazolidine: a pair of pseudoenantiomeric chiral amido-phosphine ligands. Tetrahedron 12, 695–698 (2001)
Clayden, J. & Lai, L. W. Enantioselective synthesis of atropisomeric amides by dynamic resolution: thermodynamic control with a proline-derived diamine resolving agent. Angew. Chem. Intl Edn 38, 2556–2558 (1999)
Clayden, J., Pink, J. H. & Yasin, S. A. Conformationally interlocked amides: remote asymmetric induction by mechanical transfer of stereochemical information. Tetrahedron Lett. 39, 105–108 (1998)
Clayden, J., Kenworthy, M. N. & Youssef, L. H. Axial chirality in xanthene-4,5-dicarboxamides: 1,9-stereocontrol mediated by remote interactions between conformationally constrained amide groups. Tetrahedron Lett. 41, 5171–5174 (2000)
Clayden, J., Lund, A. & Youssef, L. H. Conformational preference and remote (1,10) stereocontrol in biphenyl-2,2′-dicarboxamides. Org. Lett. 3, 4133–4136 (2001)
Clayden, J. Stereocontrol with rotationally restricted amides. Synlett, 810–816 (1998)
Krauss, R. & Koert, U. Molecular signal transduction by conformational transmission. Synlett, 598–608 (2003)
Balzani, V., Credi, A. & Venturi, M. The bottom-up approach to molecular-level devices and machines. Chem. Eur. J. 8, 5524–5542 (2002)
Sauvage, J.-P. Transition-metal containing rotaxanes and catenanes in motion: toward molecular machines and motors. Acc. Chem. Res. 31, 611–619 (1998)
Cuyegkeng, M. A. & Mannschreck, A. Chromatographic separation of enantiomers and barriers to enantiomerization of axially chiral aromatic carboxamides. Chem. Ber. 120, 803–809 (1987)
Bowles, P. et al. Atroposelectivity in the reactions of ortholithiated tertiary amides with aldehydes. J. Chem. Soc. Perkin Trans. 1, 2607–2616 (1997)
Ahmed, A. et al. Barriers to rotation about the chiral axis of tertiary aromatic amides. Tetrahedron 54, 13277–13294 (1998)
Snieckus, V. Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics. Chem. Rev. 90, 879–933 (1990)
Yin, J., Rainka, M. P., Zhang, X.-X. & Buchwald, S. L. A highly active Suzuki catalyst for the synthesis of sterically hindered biaryls: novel ligand coordination. J. Am. Chem. Soc. 124, 1162–1163 (2002)
Clayden, J., Lai, L. W. & Helliwell, M. Dynamic resolution of atropisomeric amides using proline-derived imidazolidines and ephedrine-derived oxazolidines. Tetrahedron 60, 4399–4412 (2004)
Clayden, J., McCarthy, C., Westlund, N. & Frampton, C. S. Atroposelective attack of nucleophiles on 2-formyl-1-naphthamides and their derivatives: chelation and non-chelation control. J. Chem. Soc. Perkin Trans. 1, 1363–1378 (2000)
Date, M. et al. Efficient 1,8- and 1,9-asymmetric inductions in the Grignard reaction of δ- and ɛ-keto esters of 1,1′-binaphthalen-2-ols with an oligoether tether as the 2′-substituent: application to the synthesis of (-)-malyngolide. J. Chem. Soc. Perkin Trans. 1, 645–653 (2001)
Tamai, Y. et al. Efficient 1,8- to 1,12-asymmetric induction in Grignard reactions of ω-keto esters by using BINOL or its 2′-oligoether derivatives as the chiral auxiliary. J. Chem. Soc. Perkin Trans. 1, 1141–1142 (1999)
Magnus, N. & Magnus, P. 1,13- and 1,14-asymmetric induction; remote control. Tetrahedron Lett. 38, 3491–3494 (1997)
Heinemann, C. & Demuth, M. Short biomimetic synthesis of a steroid by photoinduced electron transfer and remote asymmetric induction. J. Am. Chem. Soc. 121, 4894–4895 (1999)
Agami, C. & Rizk, T. Kinetic control of asymmetric induction during oxazolidine formation from (- )-ephedrine and aromatic aldehydes. Tetrahedron 41, 537–540 (1985)
Acknowledgements
We are grateful to the EPSRC for support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Information
Contains: Supplementary Figures S1-S6, showing NMR and HPLC evidence for the level of remote stereocontrol achieved in the addition reactions, Supplementary Figures S7-S8, showing X-ray crystal structures of 9 and 20, and experimental procedures and characterisation data for new compounds reported. (PDF 598 kb)
Rights and permissions
About this article
Cite this article
Clayden, J., Lund, A., Vallverdú, L. et al. Ultra-remote stereocontrol by conformational communication of information along a carbon chain. Nature 431, 966–971 (2004). https://doi.org/10.1038/nature02933
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02933
This article is cited by
-
Enantioselective fullerene functionalization through stereochemical information transfer from a self-assembled cage
Nature Chemistry (2023)
-
Direct observation of long-range chirality transfer in a self-assembled supramolecular monolayer at interface in situ
Nature Communications (2022)
-
Hierarchical communication of chirality for aromatic oligoamide sequences
Nature Communications (2021)
-
Stereoselective synthesis of a composite knot with nine crossings
Nature Chemistry (2018)
-
Enantioselective remote meta-C–H arylation and alkylation via a chiral transient mediator
Nature (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.