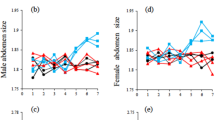
Abstract
Evolution can favour more than one reproductive tactic among conspecifics of the same sex1,2. Under the conditional evolutionarily stable strategy, individuals adopt the tactic that generates the highest fitness return for their status: large males guard females, whereas small males sneak copulations3,4. Tactics change at the status at which fitness benefits switch from favouring one tactic to favouring the alternative1,5. This ‘switchpoint’ is expressed in many species as a threshold between divergent morphologies3. Environmental and demographic parameters that influence the relative fitness of male tactics are predicted to determine a population's switchpoint1,5 and consequently whether the population is monomorphic or dimorphic. Here we show threshold evolution in the forceps dimorphism of the European earwig Forficula auricularia and document the transition from completely monomorphic to classical male-dimorphic populations over a distance of only 40 km. Because the superior fighting ability of the dominant morph6 will be more frequently rewarded at high encounter rates, population density is likely to be a key determinant of the relative fitness of the alternative tactics, and consequently the threshold. We show that, as predicted, population density correlates strongly with the shift in threshold, and that this factor drives the local evolution of the male dimorphism in these island populations. Our data provide evidence for the origin of phenotypic diversity within populations7,8,9, through the evolution of a switchpoint in a conditional strategy that has responded to local population density.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gross, M. R. Alternative reproductive tactics: diversity within sexes. Trends Ecol. Evol. 11, 92–98 (1996)
Maynard Smith, J. Evolution and the Theory of Games (Cambridge Univ. Press, Cambridge, 1982)
Hunt, J. & Simmons, L. W. Status-dependent selection in the dimorphic beetle Onthophagus taurus. Proc. R. Soc. Lond. B 268, 2409–2414 (2001)
Emlen, D. J. Alternative reproductive tactics and male dimorphism in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Behav. Ecol. Sociobiol. 41, 335–341 (1997)
Hazel, W. N., Smock, R. & Johnson, M. D. A polygenic model for the evolution and maintenance of conditional strategies. Proc. R. Soc. Lond. B 242, 181–187 (1990)
Radesäter, T. & Halldórsdóttir, H. Two male types of the common earwig: male-male competition and mating success. Ethology 95, 89–96 (1993)
West-Eberhard, M. J. Alternative adaptations, speciation and phylogeny (a review). Proc. Natl Acad. Sci. USA 83, 1388–1392 (1986)
West-Eberhard, M. J. Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 20, 249–278 (1989)
West-Eberhard, M. J. Developmental Plasticity and Evolution (Oxford, New York, 2003)
Huxley, J. S. Studies in heteronic growth (III). Discontinuous variation and heterogeny in Forficula. J. Genet. 17, 309–327 (1927)
Bateson, W. & Brindley, H. H. On some cases of variation in secondary sexual characters, statistically examined. Proc. R. Soc. Lond. 1892, 585–594 (1892)
Tomkins, J. L. Environmental and genetic determinants of the male forceps length dimorphism in the European earwig Forficula auricularia L. Behav. Ecol. Sociobiol. 47, 1–8 (1999)
Tomkins, J. L. & Simmons, L. W. Female choice and manipulations of forceps size and symmetry in the European earwig Forficula auricularia L. Anim. Behav. 56, 347–356 (1998)
Forslund, P. Male competition and large size mating advantage in the European earwigs Forficula auricularia. Anim. Behav. 59, 753–762 (2000)
Tomkins, J. L., LeBas, N. R., Unrug, J. & Radwan, J. Testing the status-dependent ESS: population variation in fighter expression in the mite Sancassania berlesei. J. Evol. Biol. (in the press)
Moczek, A. P., Hunt, J., Emlen, D. J. & Simmons, L. W. Threshold evolution in exotic populations of a polyphenic beetle. Evol. Ecol. Res. 4, 587–601 (2002)
Unrug, J., Tomkins, J. L. & Radwan, J. Alternative phenotypes and sexual selection: can dichotomous handicaps honestly signal quality? Proc. R. Soc. Lond. B 271, 1401–1406 (2004)
Roff, D. A. Evolutionary Quantitative Genetics (Chapman and Hall, New York, 1997)
Emlen, D. J. Artificial selection on horn body-length size allometry in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Evolution 50, 1219–1230 (1996)
Emlen, S. T. & Oring, L. W. Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223 (1977)
Shuster, S. M. & Wade, M. J. Mating Systems and Strategies (Princeton, Princeton, 2003)
Moczek, A. P. The behavioral ecology of threshold evolution in a polyphenic beetle. Behav. Ecol. 14, 841–854 (2004)
Roff, D. A. The evolution of threshold traits in animals. Q. Rev. Biol. 71, 3–35 (1996)
Eberhard, W. G. & Gutierrez, E. E. Male dimorphisms in beetles and earwigs and the question of developmental constraints. Evolution 45, 18–28 (1991)
Zar, J. H. Biostatistical Analysis (Prentice-Hall, New Jersey, 1984)
Kotiaho, J. S. & Tomkins, J. L. The discrimination of alternative male morphologies. Behav. Ecol. 12, 553–557 (2001)
Schluter, D. Estimating the form of natural selection on a quantitative trait. Evolution 42, 846–861 (1988)
Snow, D. W. & Perrins, C. M. Birds of the Western Palearctic Concise edition (OUP, Oxford, 1998)
Acknowledgements
We thank J. Walton, J. Stewart-Clark, H. Hamilton Dalrymple, C. Agasim-Pereira, P. Barry, K. Smith, C. Gallagher, R. Selley, B. Sampson, J. Love, D. Mawer, M. Gurr, R. Dorrien-Smith, I. Bullock, S. Avery, J. Brown and G. Thompson for permission to visit the islands; R. Mavor and D. Jones for data on seabirds; island wardens, R. Summers, J. Wilson, I. Parkinson, A. Shrieve, R. Harvey, O. Gabb, M. Steele, B. Teunis and J. Thompson for logistical support; the boat crews, B. McConnell, A. Hall (SMRU1), S. Moss, J. Dale and N. Quick (SMRU2), B. Sheil and crew (Gladtidings) and F. Mar (Sula); research assistants, A. Arthur, C. Benskin and J. Wernham; A. Arthur for artwork; K. Wilson for Splus 2000 code; N. LeBas for field assistance, discussion of the manuscript and insights into the data; and J. Alcock, N. Colegrave, D. Hosken, R. Knell, J. Kotiaho, S. Tomkins, T. Tregenza, J. Radwan, M. Ritchie, L. Simmons and K. Wilson for comments. J.L.T. is supported by a BBSRC David Phillips Research Fellowship. G.S.B. is supported by a NERC PhD studentship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Tomkins, J., Brown, G. Population density drives the local evolution of a threshold dimorphism. Nature 431, 1099–1103 (2004). https://doi.org/10.1038/nature02918
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02918
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.