Abstract
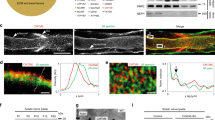
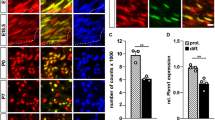
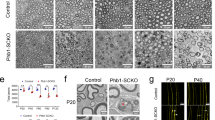
Nerve impulses are propagated at nodes of Ranvier in the myelinated nerves of vertebrates. Internodal distances have been proposed to affect the velocity of nerve impulse conduction1; however, direct evidence is lacking, and the cellular mechanisms that might regulate the length of the myelinated segments are unknown. Ramón y Cajal described longitudinal and transverse bands of cytoplasm or trabeculae in internodal Schwann cells and suggested that they had a nutritive function2. Here we show that internodal growth in wild-type nerves is precisely matched to nerve extension, but disruption of the cytoplasmic bands in Periaxin-null mice impairs Schwann cell elongation during nerve growth. By contrast, myelination proceeds normally. The capacity of wild-type and mutant Schwann cells to elongate is cell-autonomous, indicating that passive stretching can account for the lengthening of the internode during limb growth. As predicted on theoretical grounds, decreased internodal distances strikingly decrease conduction velocities and so affect motor function. We propose that microtubule-based transport in the longitudinal bands of Cajal permits internodal Schwann cells to lengthen in response to axonal growth, thus ensuring rapid nerve impulse transmission.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Brill, M. H., Waxman, S. G., Moore, J. W. & Joyner, R. W. Conduction velocity and spike configuration in myelinated fibres: computed dependence on internode distance. J. Neurol. Neurosurg. Psychiatry 40, 769–774 (1977)
Ramón y Cajal, S. Histology (Bailliere, Tindall & Cox, London, 1933)
Sherman, D. L., Fabrizi, C., Gillespie, C. S. & Brophy, P. J. Specific disruption of a Schwann cell dystrophin-related protein complex in a demyelinating neuropathy. Neuron 30, 677–687 (2001)
Gillespie, C. S. et al. Peripheral demyelination and neuropathic pain behavior in periaxin-deficient mice. Neuron 26, 523–531 (2000)
Pratt, T., Sharp, L., Nichols, J., Price, D. J. & Mason, J. O. Embryonic stem cells and transgenic mice ubiquitously expressing a tau-tagged green fluorescent protein. Dev. Biol. 228, 19–28 (2000)
Ainger, K. et al. Transport and localization elements in myelin basic protein mRNA. J. Cell Biol. 138, 1077–1087 (1997)
Carson, J. H., Worboys, K., Ainger, K. & Barbarese, E. Translocation of myelin basic protein mRNA in oligodendrocytes requires microtubules and kinesin. Cell Motil. Cytoskeleton 38, 318–328 (1997)
Colman, D. R., Kreibich, G., Frey, A. B. & Sabatini, D. D. Synthesis and incorporation of myelin polypeptides into CNS myelin. J. Cell Biol. 95, 598–608 (1982)
Griffiths, I. R. et al. Expression of myelin protein genes in Schwann cells. J. Neurocytol. 18, 345–352 (1989)
Anzini, P. et al. Structural abnormalities and deficient maintenance of peripheral nerve myelin in mice lacking the gap junction protein connexin 32. J. Neurosci. 17, 4545–4551 (1997)
Hursh, J. B. Conduction velocity and diameter of nerve fibers. Am. J. Physiol. 127, 131–139 (1939)
Huxley, A. F. & Stampfli, R. Evidence for saltatory conduction in peripheral myelinated nerve fibres. J. Physiol. (Lond.) 108, 315–339 (1949)
McIntyre, C. C., Richardson, A. G. & Grill, W. M. Modeling the excitability of mammalian nerve fibers: influence of afterpotentials on the recovery cycle. J. Neurophysiol. 87, 995–1006 (2002)
Ranvier, L. Des étranglements annulaires et des segments interannulaires chez les Raies et les Torpilles. C. R. Acad. Sci. 75, 1129–1132 (1872)
Hiscoe, H. B. Distribution of nodes and incisures in normal and regenerated nerve. Anat. Rec. 99, 447–475 (1947)
Schlaepfer, W. W. & Myers, F. K. Relationship of myelin internode elongation and growth in the rat sural nerve. J. Comp. Neurol. 147, 255–266 (1973)
Tait, S. et al. An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axo-glial junction. J. Cell Biol. 150, 657–666 (2000)
Komada, M. & Soriano, P. βIV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J. Cell Biol. 156, 337–348 (2002)
Gillespie, C. S., Sherman, D. L., Blair, G. E. & Brophy, P. J. Periaxin, a novel protein of myelinating Schwann cells with a possible role in axonal ensheathment. Neuron 12, 497–508 (1994)
Trapp, B. D. et al. Polarization of myelinating Schwann cell surface membranes: role of microtubules and the trans-Golgi network. J. Neurosci. 15, 1797–1807 (1995)
Collinson, J. M., Marshall, D., Gillespie, C. S. & Brophy, P. J. Transient expression of neurofascin by oligodendrocytes at the onset of myelinogenesis: implications for mechanisms of axon-glial interaction. Glia 23, 11–23 (1998)
Acknowledgements
We thank H. Anderson and L. Ferguson for assistance, B. Smith for technical support, S. Scherer for comments, and K. Willeke and T. Ott of Bonn University for the CMTX mice. Figure 1a is reproduced with the permission of the Cajal Institute, CSIC, Madrid, Spain, copyright inheritors of Santiago Ramón y Cajal. This work was supported by the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Court, F., Sherman, D., Pratt, T. et al. Restricted growth of Schwann cells lacking Cajal bands slows conduction in myelinated nerves. Nature 431, 191–195 (2004). https://doi.org/10.1038/nature02841
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02841
This article is cited by
-
Myelin Biology
Neurotherapeutics (2021)
-
Upregulation of large myelin protein zero leads to Charcot–Marie–Tooth disease-like neuropathy in mice
Communications Biology (2020)
-
Behind the pathology of macrophage-associated demyelination in inflammatory neuropathies: demyelinating Schwann cells
Cellular and Molecular Life Sciences (2020)
-
Nerve Conduction and Neuromuscular Transmission in C57Bl/6 Mice with Genetically Determined Peripheral Neuropathy
Neurophysiology (2019)
-
The Role of Peripheral Myelin Protein 2 in Remyelination
Cellular and Molecular Neurobiology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.