Abstract
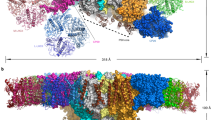
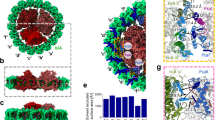
In photosynthesis, the harvesting of solar energy and its subsequent conversion into a stable charge separation are dependent upon an interconnected macromolecular network of membrane-associated chlorophyll–protein complexes. Although the detailed structure of each complex has been determined1,2,3,4, the size and organization of this network are unknown. Here we show the use of atomic force microscopy to directly reveal a native bacterial photosynthetic membrane. This first view of any multi-component membrane shows the relative positions and associations of the photosynthetic complexes and reveals crucial new features of the organization of the network: we found that the membrane is divided into specialized domains each with a different network organization and in which one type of complex predominates. Two types of organization were found for the peripheral light-harvesting LH2 complex. In the first, groups of 10–20 molecules of LH2 form light-capture domains that interconnect linear arrays of dimers of core reaction centre (RC)–light-harvesting 1 (RC–LH1–PufX) complexes; in the second they were found outside these arrays in larger clusters. The LH1 complex is ideally positioned to function as an energy collection hub, temporarily storing it before transfer to the RC where photochemistry occurs: the elegant economy of the photosynthetic membrane is demonstrated by the close packing of these linear arrays, which are often only separated by narrow ‘energy conduits’ of LH2 just two or three complexes wide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Deisenhofer, J., Epp, O., Miki, K., Huber, R. & Michel, H. Structure of the protein subunits in the photosynthetic reaction centre of Rhodopseudomonas viridis at 3 Å resolution. Nature 318, 618–624 (1985)
Allen, J. P., Feher, G., Yeates, T. O., Komiya, H. & Rees, D. C. Structure of the reaction center from Rhodobacter sphaeroides R26: The protein subunits. Proc. Natl Acad. Sci. USA 84, 6162–6166 (1987)
McDermott, G. et al. Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature 374, 517–521 (1995)
Roszak, A. W. et al. Crystal structure of the RC-LH1 core complex from Rhodopseudomonas palustris. Science 302, 1969–1972 (2003)
Walz, T., Jamieson, S. J., Bowers, C. M., Bullough, P. A. & Hunter, C. N. Projection structures of three photosynthetic complexes from Rhodobacter sphaeroides: LH2 at 6 Å, LH1 and RC-LH1 at 25 Å. J. Mol. Biol. 282, 833–845 (1998)
Jamieson, S. J. et al. Projection structure of the photosynthetic reaction centre-antenna complex of Rhodospirillum rubrum at 8.5 Å resolution. EMBO J. 21, 3927–3935 (2002)
Bahatyrova, S. et al. Flexibility and size heterogeneity of the LH1 light harvesting complex revealed by atomic force microscopy: functional significance for bacterial photosynthesis. J. Biol. Chem. 279, 21327–21333 (2004)
Fotiadis, D. et al. Structural analysis of the RC-LH1 photosynthetic core complex of Rhodospirillum rubrum using atomic force microscopy. J. Biol. Chem. 279, 2063–2068 (2004)
Siebert, C. A. et al. Molecular architecture of photosynthetic membranes in Rhodobacter sphaeroides: the role of PufX. EMBO J. 23, 690–700 (2004)
Vos, M. H., van Dorssen, R. J., Amesz, J., van Grondelle, R. & Hunter, C. N. The organisation of the photosynthetic apparatus of Rhodobacter sphaeroides: studies of antenna mutants using singlet-singlet quenching. Biochim. Biophys. Acta 933, 132–140 (1988)
Aagaard, J. & Siström, W. R. Control of synthesis of reaction centre bacteriochlorophyll in photosynthetic bacteria. Photochem. Photobiol. 15, 209–225 (1972)
Monger, T. & Parson, W. W. Singlet-triplet fusion in Rhodopseudomonas sphaeroides. Biochim. Biophys. Acta 460, 393–407 (1977)
Hunter, C. N., Kramer, H. J. M. & van Grondelle, R. Linear dichroism and fluorescence emission of antenna complexes during photosynthetic unit assembly in Rhodopseudomonas sphaeroides. Biochim. Biophys. Acta 807, 44–51 (1985)
Visscher, K. J., Bergström, H., Sundström, V., Hunter, C. N. & van Grondelle, R. Temperature-dependence of energy-transfer from the long wavelength antenna BChl-896 to the reaction centre in Rhodospirillum rubrum, Rhodobacter sphaeroides (w.t. and M21 mutant) from 77 to 177K, studied by picosecond absorption spectroscopy. Photosyn. Res. 22, 211–217 (1989)
Beekman, L. M. et al. Trapping kinetics in mutants of the photosynthetic purple bacterium Rhodobacter sphaeroides: influence of the charge separation rate and consequences for the rate-limiting step in the light-harvesting process. Biochemistry 33, 3143–3147 (1994)
Frese, R. N. et al. The long-range supraorganization of the bacterial photosynthetic unit: A key role for PufX. Proc. Natl Acad. Sci. USA 97, 5197–5202 (2000)
Scheuring, S. et al. Nanodissection and high-resolution imaging of the Rhodopseudomonas viridis photosynthetic core complex in native membranes by AFM. Proc. Natl Acad. Sci. USA 100, 1690–1693 (2003)
Hess, S. et al. Temporally and spectrally resolved subpicosecond energy transfer within the peripheral antenna complex (LH2) and from LH2 to the core antenna complex in photosynthetic purple bacteria. Proc. Natl Acad. Sci. USA 92, 12333–12337 (1995)
Scheuring, S. et al. AFM characterization of tilt and intrinsic flexibility of Rhodobacter sphaeroides light harvesting complex 2 (LH2). J. Mol. Biol. 325, 569–580 (2003)
Katona, G., Andreasson, U., Landau, E. M., Andreasson, L. E. & Neutze, R. Lipidic cubic phase crystal structure of the photosynthetic reaction centre from Rhodobacter sphaeroides at 2.35 angstrom resolution. J. Mol. Biol. 331, 681–692 (2003)
Scheuring, S. et al. Structural role of PufX in the dimerization of the photosynthetic core-complex of Rhodobacter sphaeroides. J. Biol. Chem. 279, 3620–3626 (2004)
Sturgis, J. N. & Niederman, R. A. The effect of different levels of the B800–850 light-harvesting complex on intracytoplasmic membrane development in Rhodobacter sphaeroides. Arch. Microbiol. 165, 235–242 (1996)
Müller, D. J. et al. Observing membrane protein diffusion at subnanometer resolution. J. Mol. Biol. 327, 925–930 (2003)
Berry, E. A. et al. X-ray structure of Rhodobacter capsulatus cytochrome bc1: Comparison with its mitochondrial and chloroplast counterparts. Photosyn. Res. (in the press)
Feniouk, B. A., Cherepanov, D. A., Voskoboynikova, N. E., Mulkidjanian, A. Y. & Junge, W. Chromatophore vesicles of Rhodobacter capsulatus contain on average one FoF1-ATP synthase each. Biophys. J. 82, 1115–1122 (2002)
Boekema, E. J., van Breemen, J. F., van Roon, H. & Dekker, J. P. Arrangement of photosystem II supercomplexes in crystalline macrodomains within the thylakoid membrane of green plant chloroplasts. J. Mol. Biol. 301, 1123–1133 (2000)
Niederman, R. A., Mallon, D. E. & Parks, L. C. Membranes of Rhodopseudomonas sphaeroides. VI. Isolation of a fraction enriched in newly synthesized bacteriochlorophyll α-protein complexes. Biochim. Biophys. Acta 555, 210–220 (1979)
Van der Werf, K. O. et al. Compact stand-alone atomic force microscope. Rev. Sci. Instrum. 64, 2892–2897 (1993)
Acknowledgements
This work was supported by grants from the BBSRC (UK) and the Netherlands Organisation for Scientific Research (NWO). This paper is dedicated to the memory of Prof. Dr. Bart de Grooth.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
Presents a gallery of additional images showing the arrangement of photosynthetic complexes in several membrane patches. (PDF 1278 kb)
Supplementary Figure 2
Demonstrates the very low lateral mobility of photosynthetic complexes in the membrane patches observed by AFM. (PDF 1019 kb)
Rights and permissions
About this article
Cite this article
Bahatyrova, S., Frese, R., Siebert, C. et al. The native architecture of a photosynthetic membrane. Nature 430, 1058–1062 (2004). https://doi.org/10.1038/nature02823
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02823
This article is cited by
-
Atomic force microscopic analysis of the light-harvesting complex 2 from purple photosynthetic bacterium Thermochromatium tepidum
Photosynthesis Research (2023)
-
Bioinspiration in light harvesting and catalysis
Nature Reviews Materials (2020)
-
Macroscopically Anisotropic Structures Produced by Light-induced Solvothermal Assembly of Porphyrin Dimers
Scientific Reports (2018)
-
Characterisation of a pucBA deletion mutant from Rhodopseudomonas palustris lacking all but the pucBAd genes
Photosynthesis Research (2018)
-
Energy transfer in purple bacterial photosynthetic units from cells grown in various light intensities
Photosynthesis Research (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.