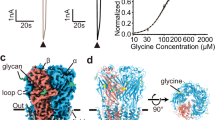
Abstract
Neurotransmitter receptors from the Cys-loop superfamily couple the binding of agonist to the opening of an intrinsic ion pore in the final step in rapid synaptic transmission. Although atomic resolution structural data have recently emerged for individual binding1 and pore domains2, how they are linked into a functional unit remains unknown. Here we identify structural requirements for functionally coupling the two domains by combining acetylcholine (ACh)-binding protein, whose structure was determined at atomic resolution1, with the pore domain from the serotonin type-3A (5-HT3A) receptor. Only when amino-acid sequences of three loops in ACh-binding protein are changed to their 5-HT3A counterparts does ACh bind with low affinity characteristic of activatable receptors, and trigger opening of the ion pore. Thus functional coupling requires structural compatibility at the interface of the binding and pore domains. Structural modelling reveals a network of interacting loops between binding and pore domains that mediates this allosteric coupling process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brejc, K. et al. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411, 269–276 (2001)
Miyazawa, A., Fujiyoshi, Y. & Unwin, N. Structure and gating mechanism of the acetylcholine receptor pore. Nature 423, 949–955 (2003)
Sine, S. M. The nicotinic receptor ligand binding domain. J. Neurobiol. 3, 431–446 (2002)
Karlin, A. Emerging structure of the nicotinic acetylcholine receptors. Nature Rev. Neurosci. 3, 102–114 (2002)
Changeux, J. P. & Edelstein, S. J. Allosteric receptors after 30 years. Neuron 21, 959–980 (1998)
Grosman, C., Salamone, F., Sine, S. M. & Auerbach, A. The extracellular linker of muscle acetylcholine receptors is a gating control element. J. Gen. Physiol. 116, 327–339 (2000)
Kash, T., Jenkins, A., Kelly, J., Trudell, J. & Harrison, N. L. Coupling of agonist binding to channel gating in the GABAA receptor. Nature 421, 272–275 (2003)
Eisele, J. L. et al. Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature 366, 479–483 (1993)
Quiram, P. & Sine, S. M. Identification of residues in the neuronal α7 receptor that confer selectivity for conotoxin ImI. J. Biol. Chem. 273, 11001–11006 (1998)
Kelley, S., Dunlop, J., Kirkness, E., Lambert, J. & Peters, J. A. A cytoplasmic region determines single-channel conductance in 5-HT3 receptors. Nature 424, 321–324 (2003)
Jackson, M. B. Perfection of a synaptic receptor: kinetics and energetics of the acetylcholine receptor. Proc. Natl Acad. Sci. USA 86, 2199–2203 (1989)
Unwin, N. Acetylcholine receptor channel imaged in the open state. Nature 373, 37–43 (1995)
Unwin, N., Miyazawa, A., Li, J. & Fujiyoshi, Y. Activation of nicotinic acetylcholine receptor involves a switch in conformation of the alpha subunits. J. Mol. Biol. 319, 1165–1176 (2002)
Hansen, S. B. et al. Tryptophan fluorescence reveals conformational changes in the acetylcholine binding protein. J. Biol. Chem. 277, 41299–41302 (2002)
Lee, B. S., Gunn, R. B. & Kopito, R. R. Functional differences among nonerythroid anion exchangers expressed in a transfected human cell line. J. Biol. Chem. 266, 11448–11454 (1991)
Gao, F. et al. Curariform antagonists bind in different orientations to acetylcholine binding protein. J. Biol. Chem. 278, 23020–23026 (2003)
Sine, S. M. Molecular dissection of subunit interfaces in the acetylcholine receptor: identification of residues that determine curare selectivity. Proc. Natl Acad. Sci. USA 90, 9436–9440 (1993)
Pear, W. S., Nolan, G. P., Scott, M. L. & Baltimore, D. Production of high titer helper-free retroviruses by transient transfection. Proc. Natl Acad. Sci. USA 90, 8392–8396 (1993)
Sine, S. M. & Taylor, P. Functional consequences of agonist-mediated state transitions in the cholinergic receptor. J. Biol. Chem. 254, 3315–3325 (1979)
Prince, R. J. & Sine, S. M. Molecular dissection of subunit interfaces in the acetylcholine receptor: identification of residues that determine agonist selectivity. J. Biol. Chem. 271, 25770–25777 (1996)
Wang, H.-L. et al. Acetylcholine receptor M3 domain: stereochemical and volume contributions to channel gating. Nature Neurosci. 2, 226–233 (1999)
Sigworth, F. & Sine, S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys. J. 52, 1047–1054 (1987)
Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391, 85–100 (1981)
Liu, Y. & Dilger, J. P. Opening rate of acetylcholine receptor channels. Biophys. J. 60, 424–432 (1991)
Spitzmaul, G., Dilger, J. P. & Bouzat, C. The noncompetitive inhibitor quinacrine modifies the desensitization kinetics of muscle acetylcholine receptors. Mol. Pharmacol. 60, 235–243 (2001)
Sali, A. & Blundell, T. L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993)
Sine, S. M., Wang, H.-L. & Bren, N. Lysine scanning mutagenesis delineates structural model of the nicotinic receptor ligand binding domain. J. Biol. Chem. 277, 29210–29223 (2002)
Acknowledgements
This work was supported by grants from the National Institutes of Health, UNS, ANPCyT and F. Antorchas, Argentina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Bouzat, C., Gumilar, F., Spitzmaul, G. et al. Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature 430, 896–900 (2004). https://doi.org/10.1038/nature02753
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02753
This article is cited by
-
The diverse family of Cys-loop receptors in Caenorhabditis elegans: insights from electrophysiological studies
Biophysical Reviews (2023)
-
Origin of acetylcholine antagonism in ELIC, a bacterial pentameric ligand-gated ion channel
Communications Biology (2022)
-
Trapping of ivermectin by a pentameric ligand-gated ion channel upon open-to-closed isomerization
Scientific Reports (2017)
-
Cholinergic receptors: functional role of nicotinic ACh receptors in brain circuits and disease
Pflügers Archiv - European Journal of Physiology (2013)
-
Molecular mechanisms of acetylcholine receptor–lipid interactions: from model membranes to human biology
Biophysical Reviews (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.