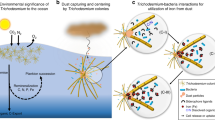
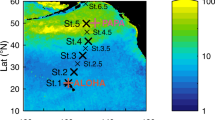
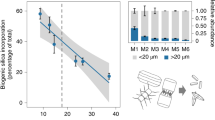
Abstract
The deposition of atmospheric dust into the ocean has varied considerably over geological time1,2. Because some of the trace metals contained in dust are essential plant nutrients which can limit phytoplankton growth in parts of the ocean, it has been suggested that variations in dust supply to the surface ocean might influence primary production3,4. Whereas the role of trace metal availability in photosynthetic carbon fixation has received considerable attention, its effect on biogenic calcification is virtually unknown. The production of both particulate organic carbon and calcium carbonate (CaCO3) drives the ocean's biological carbon pump. The ratio of particulate organic carbon to CaCO3 export, the so-called rain ratio, is one of the factors determining CO2 sequestration in the deep ocean. Here we investigate the influence of the essential trace metals iron and zinc on the prominent CaCO3-producing microalga Emiliania huxleyi. We show that whereas at low iron concentrations growth and calcification are equally reduced, low zinc concentrations result in a de-coupling of the two processes. Despite the reduced growth rate of zinc-limited cells, CaCO3 production rates per cell remain unaffected, thus leading to highly calcified cells. These results suggest that changes in dust deposition can affect biogenic calcification in oceanic regions characterized by trace metal limitation, with possible consequences for CO2 partitioning between the atmosphere and the ocean.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Petit, J. R. et al. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature 399, 429–436 (1999)
Mahowald, N. et al. Dust sources and deposition during the last glacial maximum and current climate: A comparison of model results with paleodata from ice cores and marine sediments. J. Geophys. Res. 104, 15895–15916 (1999)
Martin, J. H. Glacial-interglacial CO2 change: the iron hypothesis. Paleoceanogr. 5, 1–13 (1990)
Morel, F. M. M. et al. Zinc and carbon co-limitation of marine phytoplankton. Nature 369, 740–742 (1994)
Broecker, W. S. & Peng, T.-H. The role of CaCO3 compensation in the glacial to interglacial atmospheric CO2 change. Glob. Biogeochem. Cycles 1, 15–29 (1987)
Milliman, J. D. Production and accumulation of calcium carbonate in the ocean: budget of a nonsteady state. Glob. Biogeochem. Cycles 7, 927–957 (1993)
Baar de, H. J. W. & Boyd, P. W. in The Changing Ocean Carbon Cycle (eds Hanson, R. B., Ducklow, H. W. & Fields, J. G.) 61–140 (Cambridge Univ. Press, Cambridge, 2000)
Conkright, M. E., Levitus, S. & Boyer, T. P. World Ocean Atlas 1994, Vol. 1 Nutrients (NOAA Atlas NESDIS 1, US Department of Commerce, Washington DC, 1994)
Coale, K. H. Effects of iron, manganese, copper, and zinc enrichments on productivity and biomass in the subarctic Pacific. Limnol. Oceanogr. 36, 1851–1864 (1991)
Crawford, D. W. et al. Influence of zinc and iron enrichments on phytoplankton growth in the northeastern subarctic Pacific. Limnol. Oceanogr. 48, 1583–1600 (2003)
Sunda, W. G. & Huntsman, S. A. Cobalt and zinc interreplacement in marine phytoplankton: Biological and geochemical implications. Limnol. Oceanogr. 40, 1404–1417 (1995)
Ellwood, M. J. & Van den Berg, C. M. G. Determination of organic complexation of cobalt in seawater by cathodic stripping voltammetry. Mar. Chem. 75, 33–47 (2001)
Riebesell, U. et al. Reduced calcification of marine plankton in response to increased atmospheric CO2 . Nature 407, 364–367 (2000)
Zondervan, I., Rost, B. & Riebesell, U. Effect of CO2 concentration on the PIC/POC ratio in the coccolithophore Emiliania huxleyi grown under light-limiting conditions and different daylengths. J. Exp. Mar. Biol. Ecol. 272, 55–70 (2002)
Broecker, W. S. & Peng, T.-H. Tracers in the Sea (Eldigio, New York, 1982)
Bruland, K. W. Complexation of zinc by natural organic ligands in the central North Pacific. Limnol. Oceanogr. 34, 269–285 (1989)
Kremling, K. & Streu, P. The behaviour of dissolved Cd, Co, Zn and Pb in North Atlantic near-surface waters (30°N/60°W–60°N/2°W). Deep-Sea Res. I 48, 2541–2567 (2001)
Lohan, M. C., Statham, P. J. & Crawford, D. W. Total dissolved zinc in the upper water column of the subarctic North East pacific. Deep-Sea Res. II 49, 5793–5808 (2002)
Duce, R. et al. The atmospheric input of trace species to the world ocean. Glob. Biogeochem. Cycles 5, 193–259 (1991)
Liu, X. & Millero, F. J. The solubility of iron in seawater. Mar. Chem. 77, 43–54 (2002)
DOE. Handbook of Methods for the Analysis of the Various Parameters of the Carbon Dioxide System in Seawater (eds Dickson, A. G. & Goyet, C.) Version 2.1 ORNL/CDIAC-74 〈http://andrew.ucsd.edu/co2qc/handbook.html〉 (1994)
Pettit, L. D. & Powell, K. J. IUPAC Stability Constants Database (IUPAC and Academic Software, Otley, 2001)
Millero, F. J. & Pierrot, D. A chemical equilibrium model for natural waters. Aquat. Geochem. 4, 153–199 (1998)
Sunda, W. & Huntsman, S. Effect of pH, light, and temperature on Fe-EDTA chelation and Fe hydrolysis in seawater. Mar. Chem. 84, 35–47 (2003)
Mehrbach, C., Culberson, C. H., Hawley, J. E. & Pytkowicz, R. N. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907 (1973)
Dickson, A. G. & Millero, F. J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. 34, 1733–1743 (1987)
Stoll, M. H. C., Bakker, K., Nobbe, G. H. & Haese, R. R. Continuous flow analysis of dissolved inorganic carbon content in seawater. Anal. Chem. 73, 4111–4116 (2001)
Candelone, J.-P., Hong, S., Pellone, C. & Boutron, C. F. Post-Industrial Revolution changes in large-scale atmospheric pollution of the northern hemisphere by heavy metals as documented in central Greenland snow and ice. J. Geophys. Res. 100, 16605–16616 (1995)
Hong, S., Candelone, J.-P. & Boutron, C. F. Changes in zinc and cadmium concentrations in Greenland ice during the past 7760 years. Atmos. Environ. 31, 2235–2242 (1997)
Hong, S., Candelone, J.-P., Turetta, C. & Boutron, C. F. Changes in natural lead, copper, zinc and cadmium concentrations in central Greenland ice from 8250–149,100 years ago: their association with climate changes and resultant variations of dominant source contributions. Earth Planet. Sci. Lett. 143, 233–244 (1996)
Acknowledgements
We thank A. Terbrüggen, K.-U. Richter and B. van der Wagt for laboratory assistance, and M. Lohan, K. W. Bruland and R. E. Zeebe for discussions during the preparation of this manuscript. This work was partly funded by the German Research Foundation (DFG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Schulz, K., Zondervan, I., Gerringa, L. et al. Effect of trace metal availability on coccolithophorid calcification. Nature 430, 673–676 (2004). https://doi.org/10.1038/nature02631
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02631
This article is cited by
-
X-ray nanotomography of coccolithophores reveals that coccolith mass and segment number correlate with grid size
Nature Communications (2019)
-
Iron requirements and uptake strategies of the globally abundant marine ammonia-oxidising archaeon, Nitrosopumilus maritimus SCM1
The ISME Journal (2019)
-
Independence of nutrient limitation and carbon dioxide impacts on the Southern Ocean coccolithophore Emiliania huxleyi
The ISME Journal (2017)
-
A Genome-Wide Association Study Identifies the Genomic Region Associated with Shell Color in Yesso Scallop, Patinopecten yessoensis
Marine Biotechnology (2017)
-
Modelling how incorporation of divalent cations affects calcite wettability–implications for biomineralisation and oil recovery
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.