Abstract
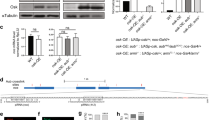
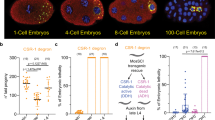
oskar messenger RNA localization at the posterior pole of the Drosophila oocyte is essential for germline and abdomen formation in the future embryo1,2. The nuclear shuttling proteins Y14/Tsunagi and Mago nashi are required for oskar mRNA localization, and they co-localize with oskar mRNA at the posterior pole of the oocyte3,4,5. Their human homologues, Y14/RBM8 and Magoh, are core components of the exon–exon junction complex (EJC)6,7,8,9. The EJC is deposited on mRNAs in a splicing-dependent manner, 20–24 nucleotides upstream of exon–exon junctions, independently of the RNA sequence6,7,8. This indicates a possible role of splicing in oskar mRNA localization, challenging the established notion that the oskar 3′ untranslated region (3′UTR) is sufficient for this process. Here we show that splicing at the first exon–exon junction of oskar RNA is essential for oskar mRNA localization at the posterior pole. We revisit the issue of sufficiency of the oskar 3′UTR for posterior localization and show that the localization of unrelated transcripts bearing the oskar 3′UTR is mediated by endogenous oskar mRNA. Our results reveal an important new function for splicing: regulation of messenger ribonucleoprotein complex assembly and organization for mRNA cytoplasmic localization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ephrussi, A., Dickinson, L. K. & Lehmann, R. Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell 66, 37–50 (1991)
Kim-Ha, J., Smith, J. L. & Macdonald, P. M. oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell 66, 23–35 (1991)
Hachet, O. & Ephrussi, A. Drosophila Y14 shuttles to the posterior of the oocyte and is required for oskar mRNA transport. Curr. Biol. 11, 1666–1674 (2001)
Mohr, S. E., Dillon, S. T. & Boswell, R. E. The RNA-binding protein Tsunagi interacts with Mago Nashi to establish polarity and localize oskar mRNA during Drosophila oogenesis. Genes Dev. 15, 2886–2899 (2001)
Newmark, P. A. & Boswell, R. E. The mago nashi locus encodes an essential product required for germ plasm assembly in Drosophila. Development 120, 1303–1313 (1994)
Kataoka, N., Diem, M. D., Kim, V. N., Yong, J. & Dreyfuss, G. Magoh, a human homolog of Drosophila mago nashi protein, is a component of the splicing-dependent exon–exon junction complex. EMBO J. 20, 6424–6433 (2001)
Le Hir, H., Izaurralde, E., Maquat, L. E. & Moore, M. J. The spliceosome deposits multiple proteins 20–24 nucleotides upstream of mRNA exon–exon junctions. EMBO J. 19, 6860–6869 (2000)
Kim, V. N. et al. The Y14 protein communicates to the cytoplasm the position of exon–exon junctions. EMBO J. 20, 2062–2068 (2001)
Le Hir, H., Gatfield, D., Braun, I. C., Forler, D. & Izaurralde, E. The protein Mago provides a link between splicing and mRNA localization. EMBO Rep. 2, 1119–1124 (2001)
Vanzo, N. F. & Ephrussi, A. Oskar anchoring restricts pole plasm formation to the posterior of the Drosophila oocyte. Development 129, 3705–3714 (2002)
Glotzer, J. B., Saffrich, R., Glotzer, M. & Ephrussi, A. Cytoplasmic flows localize injected oskar RNA in Drosophila oocytes. Curr. Biol. 7, 326–337 (1997)
Rongo, C., Gavis, E. R. & Lehmann, R. Localization of oskar RNA regulates oskar translation and requires Oskar protein. Development 121, 2737–2746 (1995)
Markussen, F. H., Michon, A. M., Breitwieser, W. & Ephrussi, A. Translational control of oskar generates short OSK, the isoform that induces pole plasma assembly. Development 121, 3723–3732 (1995)
Kim-Ha, J., Kerr, K. & Macdonald, P. M. Translational regulation of oskar mRNA by bruno, an ovarian RNA-binding protein, is essential. Cell 81, 403–412 (1995)
Kim-Ha, J., Webster, P. J., Smith, J. L. & Macdonald, P. M. Multiple RNA regulatory elements mediate distinct steps in localization of oskar mRNA. Development 119, 169–178 (1993)
Gunkel, N., Yano, T., Markussen, F. H., Olsen, L. C. & Ephrussi, A. Localization-dependent translation requires a functional interaction between the 5′ and 3′ ends of oskar mRNA. Genes Dev. 12, 1652–1664 (1998)
Ferrandon, D., Koch, I., Westhof, E. & Nusslein-Volhard, C. RNA-RNA interaction is required for the formation of specific bicoid mRNA 3′ UTR-STAUFEN ribonucleoprotein particles. EMBO J. 16, 1751–1758 (1997)
Wagner, C. et al. Dimerization of the 3′UTR of bicoid mRNA involves a two-step mechanism. J. Mol. Biol. 313, 511–524 (2001)
Palacios, I., Gatfield, D., St Johnston, D. & Izaurralde, E. An eIF4AIII-containing complex required for mRNA localization and non-sense-mediated decay. Nature 427, 753–757 (2004)
van Eeden, F. J., Palacios, I. M., Petronczki, M., Weston, M. J. & St Johnston, D. Barentsz is essential for the posterior localization of oskar mRNA and colocalizes with it to the posterior pole. J. Cell Biol. 154, 511–524 (2001)
Gatfield, D., Unterholzner, L., Ciccarelli, F. D., Bork, P. & Izaurralde, E. Nonsense-mediated mRNA decay in Drosophila: at the intersection of the yeast and mammalian pathways. EMBO J. 22, 3960–3970 (2003)
Fribourg, S., Gatfield, D., Izaurralde, E. & Conti, E. A novel mode of RBD-protein recognition in the Y14–Mago complex. Nature Struct. Biol. 10, 433–439 (2003)
Gehring, N. H., Neu-Yilik, G., Schell, T., Hentze, M. W. & Kulozik, A. E. Y14 and hUpf3b form an NMD-activating complex. Mol. Cell 11, 939–949 (2003)
Macchi, P. et al. Barentsz, a new component of the Staufen-containing ribonucleoprotein particles in mammalian cells, interacts with Staufen in an RNA-dependent manner. J. Neurosci. 23, 5778–5788 (2003)
Rørth, P. Gal4 in the Drosophila female germline. Mech. Dev. 78, 113–118 (1998)
Horton, R. M., Cai, Z. L., Ho, S. N. & Pease, L. R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 5, 528–535 (1991)
Ausubel, F. M. et al. (eds) Current Protocols in Molecular Biology vol. 1 (Wiley, New York, 1997)
Riechmann, V., Gutierrez, G. J., Filardo, P., Nebreda, A. R. & Ephrussi, A. Par-1 regulates stability of the posterior determinant Oskar by phosphorylation. Nature Cell Biol. 4, 337–342 (2002)
Wilkie, G. S., Shermoen, A. W., O'Farrell, P. H. & Davis, I. Transcribed genes are localized according to chromosomal position within polarized Drosophila embryonic nuclei. Curr. Biol. 9, 1263–1266 (1999)
Lehmann, R. & Nusslein-Volhard, C. Abdominal segmentation, pole cell formation, and embryonic polarity require the localized activity of oskar, a maternal gene in Drosophila. Cell 47, 141–152 (1986)
Acknowledgements
We thank A.-M. Voie for embryo injections for transgenesis, S. Curado for the oskA87, Nanos-Gal4:VP16 recombinant stock, A. Cyrklaff for the oskar in situ probe, P. Rørth for the pCog-Gal4:VP16 driver, D. St Johnston for anti-Staufen antibody, and members of the Ephrussi laboratory, V. Hachet and E. Izaurralde for advice and comments on the manuscript. O.H. was supported in part by a predoctoral ‘allocation de recherche’ from the French government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
oskΔi1 mRNA introns 2 and 3 are correctly spliced. (PDF 78 kb)
Rights and permissions
About this article
Cite this article
Hachet, O., Ephrussi, A. Splicing of oskar RNA in the nucleus is coupled to its cytoplasmic localization. Nature 428, 959–963 (2004). https://doi.org/10.1038/nature02521
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02521
This article is cited by
-
Comprehensive mapping of exon junction complex binding sites reveals universal EJC deposition in Drosophila
BMC Biology (2023)
-
Subcellular spatial transcriptomics identifies three mechanistically different classes of localizing RNAs
Nature Communications (2022)
-
Exon Junction Complexes can have distinct functional flavours to regulate specific splicing events
Scientific Reports (2018)
-
Adenylyl cyclase mRNA localizes to the posterior of polarized DICTYOSTELIUM cells during chemotaxis
BMC Cell Biology (2017)
-
From mechanisms to therapy: RNA processing’s impact on human genetics
Human Genetics (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.