Abstract
Myocardial infarction (MI) has become one of the leading causes of death in the world. Its pathogenesis includes chronic formation of plaque inside the vessel wall of the coronary artery and acute rupture of the artery, implicating a number of inflammation-mediating molecules, such as the cytokine lymphotoxin-α (LTA)1. Functional variations in LTA are associated with susceptibility to MI2. Here we show that LTA protein binds to galectin-2, a member of the galactose-binding lectin family3. Our case–control association study in a Japanese population showed that a single nucleotide polymorphism in LGALS2 encoding galectin-2 is significantly associated with susceptibility to MI. This genetic substitution affects the transcriptional level of galectin-2 in vitro, potentially leading to altered secretion of LTA, which would then affect the degree of inflammation; however, its relevance to other populations remains to be clarified. Smooth muscle cells and macrophages in the human atherosclerotic lesions expressed both galectin-2 and LTA. Our findings thus suggest a link between the LTA cascade and the pathogenesis of MI.
Similar content being viewed by others
Main
To understand better the role of LTA in the pathogenesis of this disease, we searched for proteins that interact with LTA. Using the Escherichia coli two-hybrid system and the phage display method, we identified galectin-2 as a binding partner of LTA. We purified the two recombinant proteins using a bacterial expression system, and confirmed direct binding of galectin-2 to LTA using an in vitro binding assay (Fig. 1a). We further examined their interaction in mammalian cells using constructs designed to express Flag-tagged LTA or S-tagged galectin-2 and western blot analysis; co-immunoprecipitation of galectin-2 and LTA confirmed their interaction (Fig. 1b). Using antibodies specific to each protein, we also investigated subcellular localization of native galectin-2 and LTA in U937 cells, and found that these proteins co-localized in the cytoplasm (Fig. 1c).
We then examined whether any genetic variation in LGALS2 was associated with susceptibility to MI. Re-sequencing the LGALS2 genomic region using DNA from 32 MI samples revealed 17 additional single nucleotide polymorphisms (SNPs) (Fig. 2a). Next, we compared the genotype frequencies of approximately 600 individuals with MI, and 600 controls at these SNP loci, and found that one SNP (3279C → T) in intron-1 of LGALS2 was significantly associated with MI (designated SNP9 in Supplementary Table 1). This association was confirmed by increasing the number of samples to 2,302 for patients with MI and 2,038 for controls, and by including another set of samples (Table 1 and Supplementary Table 2).
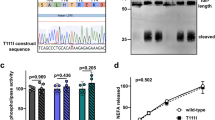
a, Map of SNPs in LGALS2 locus. b, c, Transcriptional regulatory activity of intron-1 SNP of LGALS2 in HeLa (b) and HepG2 (c) cells. d–f, Inhibition of galectin-2 expression. Levels of galectin-1 or galectin-2 mRNA (d), supernatant LTA protein (e), and LTA mRNA (f) after 48 h transfection with siRNA vectors. *Student's t-test. Each experiment was repeated three times and each sample was studied in triplicate.
We also analysed linkage disequilibrium using a subset of markers with minor allele frequency of >0.20, and investigated haplotype structure within the LGALS2 region (Supplementary Table 3). This allowed us to identify a haplotype block containing three SNPs (SNP9, SNP10 and SNP11); no particular haplotype showed a higher statistical significance for association with MI than the single SNP alone (Supplementary Table 4). Because the minor allele frequency of the associated SNP was lower in the patients' group (Table 1), we concluded from these genetic studies that the minor variant protects against the risk of MI, although our study included only Japanese subjects and so its relevance to other populations remains to be clarified.
To investigate the biological significance of this genetic variation in intron-1 of LGALS2, we constructed reporter plasmids with a genomic fragment containing the SNP downstream of a luciferase gene and the SV40 enhancer sequence, and examined the effect of the intron-1 SNP on gene expression. The clone containing the 3279T allele showed nearly 50% less transcriptional activity than those containing the 3279C allele or the vector alone (Fig. 2b, c). These observations indicated that the nucleotide substitution could potentially reduce the level of the galectin-2 transcript.
We then hypothesized that the amount of intracellular galectin-2 might regulate the extracellular secretion level of LTA, thereby influencing the degree of inflammation. To test this hypothesis, we examined the effect of the level of galectin-2 on the secreted level of LTA in the medium by repressing galectin-2 expression using a small interference (si)RNA technique, and by over-expressing galectin-2. One siRNA for galectin-2 repressed galectin-2 messenger RNA to nearly one-fifth of its original level (Fig. 2d) and resulted in inhibition of LTA secretion into the medium (Fig. 2e). Over-expression of galectin-2 enhanced LTA secretion (Supplementary Fig. 1a). As shown in Fig. 2f, the LTA mRNA level was unchanged by knockdown or over-expression of galectin-2 (see also Supplementary Fig. 1b).
To further investigate the regulatory mechanism of LTA secretion by galectin-2, we searched for intracellular molecules that associate with galectin-2, using a tandem affinity purification (TAP) system4. We identified two unique bands that could be observed only when the galectin-2-TAP tag was expressed (Fig. 3a). On the basis of a MALDI/TOF (matrix-assisted laser desorption/ionization–time of flight) mass spectrometry analysis, these two bands were shown to correspond to α- and β-tubulins—important components of microtubules. Using HeLa cells transfected with a plasmid to express Flag-tagged galectin-2, we confirmed co-immunoprecipitation of endogenous tubulins and galactin-2 (Fig. 3b and data not shown). Interestingly, the tubulins were also co-immunoprecipitated with LTA (Fig. 3b and data not shown). Images from serial confocal sections of double-immunostained U937 cells revealed that galectin-2 and α-tubulin co-localized as reticular filamentous networks developed in the cytoplasm (Fig. 3c). Recently, microtubule cytoskeleton networks have been implicated in the subcellular transport of some proteins, including glucose transporter isoform (GLUT4) or thiamine transporter (THTR1)5,6. It is likely that LTA is another molecule that uses the microtubule cytoskeleton network for translocation. It is also conceivable that galectin-2 has a role in intracellular trafficking, although the precise role of galectin-2 in this trafficking machinery complex has yet to be elucidated.
a, Isolation of TAP-tagged galectin-2 and interacting proteins. Arrowheads indicate α- and β-tubulins, revealed by MALDI/TOF mass spectrometry analyses. b, Co-immunoprecipitation of endogenous α-tubulin with Flag-tagged galectin-2 or LTA. Immunoprecipitations were done using anti-Flag M2 agarose, and immunoprecipitates were detected using anti-α-tubulin antibody (upper panel) or anti-Flag antibody (lower panel). Flag-tagged LacZ encoding β-galactosidase was used as a negative control. c, Co-localization of endogenous α-tubulin with endogenous galectin-2 or LTA in U937 cells.
To examine whether these proteins are expressed in the lesion of MI—that is, in atherosclerotic lesions of the coronary artery—and if so, to investigate in which part of the lesion they are expressed, we performed immunohistochemical staining of human coronary atherectomy specimens with anti-LTA or anti-galectin-2 antibody. As shown in Fig. 4a, b, immunoreactivities for both LTA and galectin-2 were detected in intimal cells in atherosclerotic plaques, some of which were spindle-shaped or contained vacuolated, round cytoplasms. Immunostaining of adjacent sections with anti-smooth muscle cell (SMC) α-actin or anti-CD68 showed that the majority of these cells were either SMCs or SMC-derived foam cells, with occasional macrophages being observed (Fig. 4c, d and Supplementary Fig. 2). Co-expression of LTA and galectin-2 was also observed in the majority of polymorphic SMCs by double-labelled immunohistochemistry (Fig. 4e). In contrast, expression of either protein was not detectable in atrophic SMCs of fibrous plaques with scanty cellularity or in normal medial SMCs (Fig. 4f for LTA; data not shown for galectin-2).
Single-labelled immunohistochemistry of serial sections of primary atherosclerotic lesions from human coronary arteries obtained by directional coronary atherectomy, stained with anti-human LTA (a), anti-human galectin-2 (b), monoclonal anti-SMC α-actin (c), and monoclonal anti-CD68 (d). Magnification, ×100. e, Double-labelled immunohistochemical staining with anti-LTA (brown) and galectin-2 (blue) antibodies. Magnification, ×119. f, Single-labelled immunohistochemical staining with anti-human LTA for atrophic SMCs of fibrous plaques with scanty cellularity and normal medial SMCs. Magnification, ×100.
These results showed that LTA and galectin-2 were co-expressed in SMCs and macrophages in the intima of human atherosclerotic plaques, but were absent in quiescent or normal medial SMCs. We also confirmed their co-expression by counting immunohistochemically double-labelled cells (Supplementary Table 5). A recent report also indicated that LTA was expressed in atherosclerotic lesions in mice and that loss of LTA reduced the size of the lesions7. Together, these results indicate that LTA, as one of the mediators of inflammation, along with galectin-2, as a regulator of LTA secretion, might have roles in the pathogenesis of MI, although the functional correlation of galectin-2 and LTA with the development, progression or rupture of atherosclerotic plaques has yet to be clarified.
We have identified galectin-2 as another risk factor for MI. We believe that the results of this study provide an anchoring point for better understanding of the pathogenesis of MI.
Methods
DNA samples
The study included 2,638 Japanese patients with MI, referred to as the Osaka Acute Coronary Insufficiency Study (OACIS) group. The diagnosis of definite MI has been described previously2. The control subjects consisted of 2,499 general populations recruited through several medical institutes in Japan. All subjects were Japanese and gave written informed consent to participation in the study; or if they were under 20 years old, their parents gave consent according to the process approved by the Ethical Committee at the SNP Research Center, The Institute of Physical and Chemical Research (RIKEN), Yokohama.
SNP analysis
Design for polymerase chain reaction (PCR) primers, PCR experiments, DNA extraction, DNA sequencing, SNP discovery, genotyping of SNPs and statistical analysis has been described previously8,9,10.
E. coli two-hybrid and phage display screening
We constructed a two-hybrid complementary DNA (cDNA) library using mRNA isolated from Jurkat cells (RIKEN Cell Bank; RCB0806) and BacterioMatch two-hybrid system library construction kit (Clontech), and screened the library using pBT-human LTA as the ‘bait’. For the phage display system, we constructed a phage display cDNA library using mRNA isolated from Jurkat cells and T7 select cDNA cloning system (Novagen). We screened the library using the immobilized LTA as the ‘bait’.
Tandem affinity purification
The tandem affinity purification procedure was carried out essentially as described in ref. 4, with some modifications. We constructed a fusion cassette encoding a His tag, a TEV cleavage site, and an S tag as a TAP tag sequence in pCMV–Myc vector (Sigma). This TAP vector expresses amino-terminal Myc-tagged target protein with carboxy-terminal TAP tags in mammalian cells under the control of cytomegalovirus promoter. The TAP vector was transiently transfected into HeLa cells (Health Science Research Resources Bank (HSRRB); JCRB9004). Target protein bands were analysed by MALDI/TOF mass spectrometry at APRO Life Science.
In vitro binding assay and co-immunoprecipitation experiment
We prepared purified S-tagged recombinant LTA and T7-tagged galectin-2 derived from E. coli using the pET system (Novagen), and combined them. The co-immunoprecipitation experiments were performed using a monoclonal antibody against LTA (R&D Systems) coupled to HiTrapTM NHS-activated Sepharose HP (Amersham). We visualized the immune complex using T7 tag antibody (Stratagene) and horseradish peroxidase (HRP) conjugated with anti-mouse IgG antibody. For co-immunoprecipitation in mammalian cells, we transfected expression plasmids of Flag or S-tagged LTA, galectin-2 and LacZ (as a negative control) into COS7 cells (HSRRB; JCRB9127) or HeLa cells using Fugene. Immunoprecipitations were done in lysis buffer (20 mM Tris pH 7.5, with 150 mM NaCl, 0.1 % Nonident P-40). Twenty-four hours after transfection, cells were lysed, and immunoprecipitations were performed using anti-Flag tag M2 agarose (Sigma). We visualized the immune complex using HRP-conjugated S-protein (Novagen), anti-Flag M2 peroxidase conjugate (Sigma) or mouse monoclonal antibody against human α-tubulin (Molecular Probes) and HRP-conjugated anti-mouse IgG antibody.
Confocal microscopy
Polyclonal anti-human galectin-2 antisera were raised in rabbits using recombinant protein synthesized in E. coli. The antisera showed no cross-reactivity to structurally related molecules galectin-1 and galectin-3, analysed by western blot. Polyclonal anti-galectin-2 antisera and either goat anti-human LTA IgG (R&D Systems) or mouse anti-human α-tubulin monoclonal IgM antibodies were used with Alexa secondary antibodies (Molecular Probes). U937 cells (HSRRB; JCRB9021) were stimulated for 30 min with phorbol myristate acetate (PMA) (20 ng ml-1) and fixed. They were subsequently incubated with the corresponding primary antibodies in phosphate-buffered saline containing 3% bovine serum albumin, and the corresponding Alexa secondary antibodies.
siRNA and over-expression experiments
The target sequences for galectin-2 (5′-AATCCACCATTGTCTGCAACT-3′) were cloned into pSilencer 2.0-U6 siRNA vector (Ambion). For the over-expression experiment, the galectin-2 was cloned into pFlag–CMV5a vector. After transfection, Jurkat cells were stimulated with PMA (20 ng ml-1) for 24 h, and cells and supernatants were collected separately. LTA concentration was measured using an LTA-specific ELISA system (R&D Systems), and normalized by comparison with total protein concentration. The mRNA quantification procedure has been described previously2.
Luciferase assay
A DNA fragment, corresponding to nucleotides 3,188 to 3,404 of intron-1 of LGALS2, was cloned into pGL3–enhancer vector (Promega) in the downstream of SV40 enhancer in the 5′ to 3′ orientation. After 24 h transfection, luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega).
Immunohistochemistry
Tissue samples were obtained from 16 patients with MI by elective directional coronary atherectomy. Immunohistochemical protocols were carried out as described previously11,12 using goat anti-human LTA IgG (R&D Systems) and rabbit polyclonal anti-human galectin-2 antibody. Staining of adjacent sections was carried out using human-cell-type-specific monclonal antibodies against SMC 2–actin and CD68 (DAKO). For double-labelled immunohistochemistry, sections were incubated with anti-LTA antibody, then with biotinylated swine anti-goat IgG, and then with avidin–biotin–peroxidase conjugate, followed by visualization with 3,3′-diaminobenzidine tetrahydrochloride (Vector Labs). The section was subsequently incubated with rabbit polyclonal anti-human galectin-2 antibody, followed by incubation with alkaline phosphatase-conjugated swine anti-rabbit IgG and visualized with the 5-bromo-4-chloro-3-indoxyl phosphate and nitro-blue tetrazolium chloride (BCIP/NBT) substrate system.
References
Ross, R. Atherosclerosis—an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999)
Ozaki, K. et al. Functional SNPs in the lymphotoxin-α gene that are associated with susceptibility to myocardial infarction. Nature Genet. 32, 650–654 (2002)
Gitt, M. A., Massa, S. M., Leffler, H. & Barondes, S. H. Isolation and expression of a gene encoding L-14-II, a new human soluble lactose-binding lectin. J. Biol. Chem. 267, 10601–10606 (1992)
Rigaut, G. et al. A generic protein purification method for protein complex characterization and proteome exploration. Nature Biotechnol. 17, 1030–1032 (1999)
Liu, L. B., Omata, W., Kojima, I. & Shibata, H. Insulin recruits GLUT4 from distinct compartments via distinct traffic pathways with differential microtubule dependence in rat adipocytes. J. Biol. Chem. 278, 30157–30169 (2003)
Subramanian, V. S., Marchant, J. S., Parker, I. & Said, H. M. Cell biology of the human thiamine transporter-1 (hTHTR1). Intracellular trafficking and membrane targeting mechanisms. J. Biol. Chem. 278, 3976–3984 (2003)
Schreyer, S. A., Vick, C. M. & LeBoeuf, R. C. L. Loss of lymphotoxin-α but not tumor necrosis factor-α reduces atherosclerosis in mice. J. Biol. Chem. 277, 12364–12368 (2002)
Iida, A. et al. Catalog of 258 single-nucleotide polymorphisms (SNPs) in genes encoding three organic anion transporters, three organic anion-transporting polypeptides, and three NADH:ubiquinone oxidoreductase flavoproteins. J. Hum. Genet. 46, 668–683 (2001)
Ohnishi, Y. et al. A high-throughput SNP typing system for genome-wide association studies. J. Hum. Genet. 46, 471–477 (2001)
Yamada, R. et al. Association between a single-nucleotide polymorphism in the promoter of the human interleukin-3 gene and rheumatoid arthritis in Japanese patients, and maximum-likelihood estimation of combinatorial effect that two genetic loci have on susceptibility to the disease. Am. J. Hum. Genet. 68, 674–685 (2001)
Minami, M. et al. Expression of SR-PSOX, a novel cell-surface scavenger receptor for phosphatidylserine and oxidized LDL in human atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 21, 1796–1800 (2001)
Shi, S. R., Key, M. E. & Kalra, K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J. Histochem. Cytochem. 39, 741–748 (1991)
den Dunnen, J. T. & Antonarakis, S. E. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum. Mutat. 15, 7–12 (2000)
Acknowledgements
We thank M. Takahashi, M. Yoshii, M. Omotezako, Y. Ariji, S. Abiko, W. Yamanobe and K. Tabei for their assistance. We also thank all the other members of the SNP Research Center, RIKEN, for their contribution to the completion of our study. This work was supported by a grant from the Japanese Millennium Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
Over-expression of galectin-2 resulted in enhanced level of LTA secretion. (PDF 268 kb)
Supplementary Figure 2
Expression of galectin-2 and LTA in SMCs and macrophages in the intima of human atherosclerotic plaques. (PDF 1896 kb)
Supplementary Table 1
Summary for association analyses of 17 SNPs in LGALS2 region with myocardial infarction. (DOC 65 kb)
Supplementary Table 2
Confirmation of association of a SNP in LGALS2 with myocardial infarction. (DOC 31 kb)
Supplementary Table 3
Summary of pairwise LD coefficients among SNPs in LGALS2 region. (DOC 32 kb)
Supplementary Table 4
Association analyses of haplotypes in LGALS2 with myocardial infarction. (DOC 30 kb)
Supplementary Table 5
Confirmation of LTA and galectin-2 co-expression by cell counting of double-labeled immunohistochemistry. (DOC 26 kb)
Rights and permissions
About this article
Cite this article
Ozaki, K., Inoue, K., Sato, H. et al. Functional variation in LGALS2 confers risk of myocardial infarction and regulates lymphotoxin-α secretion in vitro. Nature 429, 72–75 (2004). https://doi.org/10.1038/nature02502
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02502
This article is cited by
-
Regulation of wound healing and fibrosis by galectins
Journal of Molecular Medicine (2022)
-
Personalized medicine in cardiovascular disease: review of literature
Journal of Diabetes & Metabolic Disorders (2021)
-
Seasonal and flight-related variation of galectin expression in heart, liver and flight muscles of yellow-rumped warblers (Setophaga coronata)
Glycoconjugate Journal (2017)
-
Long-term outdoor air pollution and DNA methylation in circulating monocytes: results from the Multi-Ethnic Study of Atherosclerosis (MESA)
Environmental Health (2016)
-
Molecular genetics of coronary artery disease
Journal of Human Genetics (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.