Abstract
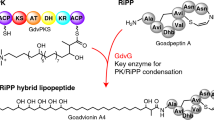
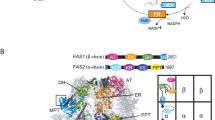
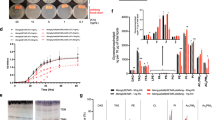
The metabolic repertoire in nature is augmented by generating hybrid metabolites from a limited set of gene products1,2,3. In mycobacteria, several unique complex lipids are produced by the combined action of fatty acid synthases and polyketide synthases (PKSs)4,5,6, although it is not clear how the covalently sequestered biosynthetic intermediates are transferred from one enzymatic complex to another. Here we show that some of the 36 annotated fadD genes, located adjacent to the PKS genes in the Mycobacterium tuberculosis genome, constitute a new class of long-chain fatty acyl-AMP ligases (FAALs). These proteins activate long-chain fatty acids as acyl-adenylates, which are then transferred to the multifunctional PKSs for further chain extension. This mode of activation and transfer of fatty acids is contrary to the previously described universal mechanism involving the formation of acyl-coenzyme A thioesters. Similar mechanisms may operate in the biosynthesis of other lipid-containing metabolites and could have implications in engineering novel hybrid products.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Moore, B. S. & Hertweck, C. Biosynthesis and attachment of novel bacterial polyketide synthase starter units. Nat. Prod. Rep. 19, 70–99 (2002)
Hopwood, D. A. Genetic contributions to understanding polyketide synthases. Chem. Rev. 97, 2465–2498 (1997)
Spaink, H. P. et al. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature 354, 125–130 (1991)
Gokhale, R. S. & Tuteja, D. in Biotechnology (eds Rehm, H.-J. & Reed, G.) 341–372 (Wiley, Weinheim, 2001)
Kolattukudy, P. E., Fernandes, N. D., Azad, A. K., Fitzmaurice, A. M. & Sirakova, T. D. Biochemistry and molecular genetics of cell-wall lipid biosynthesis in mycobacteria. Mol. Microbiol. 24, 263–270 (1997)
Sirakova, T. D., Thirumala, A. K., Dubey, V. S., Sprecher, H. & Kolattukudy, P. E. The Mycobacterium tuberculosis pks2 gene encodes the synthase for the hepta- and octamethyl-branched fatty acids required for sulfolipid synthesis. J. Biol. Chem. 276, 16833–16839 (2001)
Cole, S. T. et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 (1998)
Azad, A. K., Sirakova, T. D., Fernandes, N. D. & Kolattukudy, P. E. Gene knockout reveals a novel gene cluster for the synthesis of a class of cell wall lipids unique to pathogenic mycobacteria. J. Biol. Chem. 272, 16741–16745 (1997)
Cox, J. S., Chen, B., McNeil, M. & Jacobs, W. R. Jr Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402, 79–83 (1999)
Camacho, L. R. et al. Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. Evidence that this lipid is involved in the cell wall permeability barrier. J. Biol. Chem. 276, 19845–19854 (2001)
Rindi, L. et al. Involvement of the fadD33 gene in the growth of Mycobacterium tuberculosis in the liver of BALB/c mice. Microbiol. 148, 3873–3880 (2002)
Conti, E., Franks, N. P. & Brick, P. Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure 4, 287–298 (1996)
May, J. J., Kessler, N., Marahiel, M. A. & Stubbs, M. T. Crystal structure of DhbE, an archetype for aryl acid activating domains of modular nonribosomal peptide synthetases. Proc. Natl Acad. Sci. USA 99, 12120–12125 (2002)
Gulick, A. M., Starai, V. J., Horswill, A. R., Homick, K. M. & Escalante-Semerena, J. C. The 1.75 A crystal structure of acetyl-CoA synthetase bound to adenosine-5′-propylphosphate and coenzyme A. Biochemistry 42, 2866–2873 (2003)
Fitzmaurice, A. M. & Kolattukudy, P. E. An acyl-CoA synthase (acoas) gene adjacent to the mycocerosic acid synthase (mas) locus is necessary for mycocerosyl lipid synthesis in Mycobacterium tuberculosis var. bovis BCG. J. Biol. Chem. 273, 8033–8039 (1998)
Fitzmaurice, A. M. & Kolattukudy, P. E. Open reading frame 3, which is adjacent to the mycocerosic acid synthase gene, is expressed as an acyl coenzyme A synthase in Mycobacterium bovis BCG. J. Bacteriol. 179, 2608–2615 (1997)
Saxena, P., Yadav, G., Mohanty, D. & Gokhale, R. S. A new family of type III polyketide synthases in Mycobacterium tuberculosis. J. Biol. Chem. 278, 44780–44790 (2003)
Yadav, G., Gokhale, R. S. & Mohanty, D. SEARCHPKS: A program for detection and analysis of polyketide synthase domains. Nucleic Acids Res. 31, 3654–3658 (2003)
Yadav, G., Gokhale, R. S. & Mohanty, D. Computational approach for prediction of domain organization and substrate specificity of modular polyketide synthases. J. Mol. Biol. 328, 335–363 (2003)
Lambalot, R. H. et al. A new enzyme superfamily—the phosphopantetheinyl transferases. Chem. Biol. 3, 923–936 (1996)
Gokhale, R. S., Tsuji, S. Y., Cane, D. E. & Khosla, C. Dissecting and exploiting intermodular communication in polyketide synthases. Science 284, 482–485 (1999)
Duitman, E. H. et al. The mycosubtilin synthetase of Bacillus subtilis ATCC6633: a multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthase. Proc. Natl Acad. Sci. USA 96, 13294–13299 (1999)
Tosato, V., Albertini, A. M., Zotti, M., Sonda, S. & Bruschi, C. V. Sequence completion, identification and definition of the fengycin operon in Bacillus subtilis 168. Microbiology 143, 3443–3450 (1997)
Admiraal, S. J., Walsh, C. T. & Khosla, C. The loading module of rifamycin synthetase is an adenylation-thiolation didomain with substrate tolerance for substituted benzoates. Biochemistry 40, 6116–6123 (2001)
Polverino de Laureto, P. et al. Protein aggregation and amyloid fibril formation by an SH3 domain probed by limited proteolysis. J. Mol. Biol. 334, 129–141 (2003)
Dieckmann, R., Pavela-Vrancic, M., von Dohren, H. & Kleinkauf, H. Probing the domain structure and ligand-induced conformational changes by limited proteolysis of tyrocidine synthetase 1. J. Mol. Biol. 288, 129–140 (1999)
Keller, U., Kleinkauf, H. & Zocher, R. 4-Methyl-3-hydroxyanthranilic acid activating enzyme from actinomycin-producing Streptomyces chrysomallus. Biochemistry 23, 1479–1484 (1984)
Acknowledgements
The authors thank S. K. Basu for discussions. P.A. is a Senior Research Fellow of CSIR, India. R.S.G is a Wellcome Trust International Senior Research Fellow in India. This work was also supported by grants to the National Institute of Immunology by DBT, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
Dendrogram obtained from the multiple sequence alignment of the FadDs of M. tuberculosis. (PPT 97 kb)
Supplementary Figure 2
Exogenous incorporation of acyl-CoA. (PPT 2325 kb)
Supplementary Figure 3
SDS-PAGE gel showing purified FadD and PKS proteins used in this study. (PPT 276 kb)
Supplementary Figure Legends
SDS-PAGE gel showing purified FadD and PKS proteins used in this study. (DOC 7 kb)
Rights and permissions
About this article
Cite this article
Trivedi, O., Arora, P., Sridharan, V. et al. Enzymic activation and transfer of fatty acids as acyl-adenylates in mycobacteria. Nature 428, 441–445 (2004). https://doi.org/10.1038/nature02384
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02384
This article is cited by
-
Mapping the biosynthetic pathway of a hybrid polyketide-nonribosomal peptide in a metazoan
Nature Communications (2021)
-
Recent Insights into the Structure and Function of Mycobacterial Membrane Proteins Facilitated by Cryo-EM
The Journal of Membrane Biology (2021)
-
Tuberculosis: current scenario, drug targets, and future prospects
Medicinal Chemistry Research (2021)
-
Molecular Cloning, Purification and Characterization of Mce1R of Mycobacterium tuberculosis
Molecular Biotechnology (2021)
-
Key elements and regulation strategies of NRPSs for biosynthesis of lipopeptides by Bacillus
Applied Microbiology and Biotechnology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.