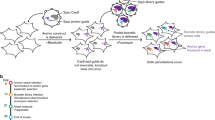
Abstract
RNA interference (RNAi) is a powerful new tool with which to perform loss-of-function genetic screens in lower organisms and can greatly facilitate the identification of components of cellular signalling pathways1,2,3. In mammalian cells, such screens have been hampered by a lack of suitable tools that can be used on a large scale. We and others have recently developed expression vectors to direct the synthesis of short hairpin RNAs (shRNAs) that act as short interfering RNA (siRNA)-like molecules to stably suppress gene expression4,5. Here we report the construction of a set of retroviral vectors encoding 23,742 distinct shRNAs, which target 7,914 different human genes for suppression. We use this RNAi library in human cells to identify one known and five new modulators of p53-dependent proliferation arrest. Suppression of these genes confers resistance to both p53-dependent and p19ARF-dependent proliferation arrest, and abolishes a DNA-damage-induced G1 cell-cycle arrest. Furthermore, we describe siRNA bar-code screens to rapidly identify individual siRNA vectors associated with a specific phenotype. These new tools will greatly facilitate large-scale loss-of-function genetic screens in mammalian cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lum, L. et al. Identification of Hedgehog pathway components by RNAi in Drosophila cultured cells. Science 299, 2039–2045 (2003)
Kamath, R. S. et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231–237 (2003)
Ashrafi, K. et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421, 268–272 (2003)
Brummelkamp, T. R., Bernards, R. & Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553 (2002)
Paddison, P. J., Caudy, A. A., Bernstein, E., Hannon, G. J. & Conklin, D. S. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 16, 948–958 (2002)
Hannon, G. J. RNA interference. Nature 418, 244–251 (2002)
Elbashir, S. M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001)
Sherr, C. J. The ink4a/ARF network in tumour suppression. Nature Rev. Mol. Cell Biol. 2, 731–737 (2001)
Brummelkamp, T., Bernards, R. & Agami, R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2, 243–247 (2002)
Brummelkamp, T. R., Nijman, S. M., Dirac, A. M. & Bernards, R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-κB. Nature 424, 797–801 (2003)
Semizarov, D. et al. Specificity of short interfering RNA determined through gene expression signatures. Proc. Natl Acad. Sci. USA 100, 6347–6352 (2003)
Jackson, A. L. et al. Expression profiling reveals off-target gene regulation by RNAi. Nature Biotechnol. 21, 635–637 (2003)
Kamijo, T. et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91, 649–659 (1997)
Lakin, N. D. & Jackson, S. P. Regulation of p53 in response to DNA damage. Oncogene 18, 7644–7655 (1999)
El-Deiry, W. S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993)
Brugarolas, J. et al. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377, 552–557 (1995)
Waldman, T., Kinzler, K. W. & Vogelstein, B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 55, 5187–5190 (1995)
Brown, J. P., Wei, W. & Sedivy, J. M. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science 277, 831–834 (1997)
Miyashita, T. & Reed, J. C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80, 293–299 (1995)
Barak, Y., Juven, T., Haffner, R. & Oren, M. mdm2 expression is induced by wild type p53 activity. EMBO J. 12, 461–468 (1993)
Kubbutat, M. H., Jones, S. N. & Vousden, K. H. Regulation of p53 stability by Mdm2. Nature 387, 299–303 (1997)
Brummelkamp, T. R. & Bernards, R. New tools for functional mammalian cancer genetics. Nature Rev. Cancer 3, 781–789 (2003)
Shoemaker, D. D., Lashkari, D. A., Morris, D., Mittmann, M. & Davis, R. W. Quantitative phenotypic analysis of yeast deletion mutants using a highly parallel molecular bar-coding strategy. Nature Genet. 14, 450–456 (1996)
Brummelkamp, T. R. et al. TBX-3, the gene mutated in Ulnar-Mammary Syndrome, is a negative regulator of p19ARF and inhibits senescence. J. Biol. Chem. 277, 6567–6572 (2002)
Kanda, T., Sullivan, K. F. & Wahl, G. M. Histone–GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 8, 377–385 (1998)
Wang, A. H. et al. HDAC4, a human histone deacetylase related to yeast HDA1, is a transcriptional corepressor. Mol. Cell. Biol. 19, 7816–7827 (1999)
Schwarz, D. S. et al. Asymmetry in the assembly of the RNAi enzyme complex. Cell 115, 199–208 (2003)
Heetebrij, R. J. et al. Platinum(II)-based coordination compounds as nucleic acid labeling reagents: synthesis, reactivity, and applications in hybridization assays. Chembiochem 4, 573–583 (2003)
Trettel, F. et al. Dominant phenotypes produced by the HD mutation in STHdh(Q111) striatal cells. Hum. Mol. Genet. 9, 2799–2809 (2000)
Dirac, A. M. & Bernards, R. Reversal of senescence in mouse fibroblasts through lentiviral suppression of p53. J. Biol. Chem. 278, 11731–11734 (2003)
Acknowledgements
We thank S. Friend and J. Downward for their support of this project, M. Voorhoeve, Z. Wu, X.-j. Yang, H. Yntema and Kreatech Biotechnology for reagents, the NKI microarray facility group for assistance, A. Dirac and S. Nijman for technical help, and members of the Bernards laboratory for discussions. This work was supported by grants from the Netherlands Genomics Initiative/Netherlands Organization for Scientific Research (NWO), Cancer Research UK (CRUK), the Centre for Biomedical Genetics (CBG), the Dutch Cancer Society (KWF) and Utrecht University (ABC cluster).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
Schematic representation of the 59 nt oligonucleotides. (PPT 21 kb)
Supplementary Figure 2
Knockdown of endogenous gene expression. (PPT 27 kb)
Supplementary Table 1
Genes in the NKI RNAi library. (XLS 1082 kb)
Supplementary Table 2
Sequence information of identified shRNA vectors. (PDF 14 kb)
Rights and permissions
About this article
Cite this article
Berns, K., Hijmans, E., Mullenders, J. et al. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 428, 431–437 (2004). https://doi.org/10.1038/nature02371
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02371
This article is cited by
-
CUL4A silencing attenuates cervical carcinogenesis and improves Cisplatin sensitivity
Molecular and Cellular Biochemistry (2023)
-
Simultaneous expression of MMB-FOXM1 complex components enables efficient bypass of senescence
Scientific Reports (2021)
-
Combinatorial CRISPR screen identifies fitness effects of gene paralogues
Nature Communications (2021)
-
CaMKK2 facilitates Golgi-associated vesicle trafficking to sustain cancer cell proliferation
Cell Death & Disease (2021)
-
Functional genomics for breast cancer drug target discovery
Journal of Human Genetics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.