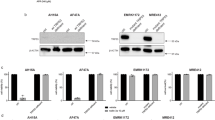
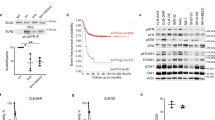
Abstract
Evading apoptosis is considered to be a hallmark of cancer, because mutations in apoptotic regulators invariably accompany tumorigenesis1. Many chemotherapeutic agents induce apoptosis, and so disruption of apoptosis during tumour evolution can promote drug resistance2. For example, Akt is an apoptotic regulator that is activated in many cancers and may promote drug resistance in vitro3. Nevertheless, how Akt disables apoptosis and its contribution to clinical drug resistance are unclear. Using a murine lymphoma model, we show that Akt promotes tumorigenesis and drug resistance by disrupting apoptosis, and that disruption of Akt signalling using the mTOR inhibitor rapamycin reverses chemoresistance in lymphomas expressing Akt, but not in those with other apoptotic defects. eIF4E, a translational regulator that acts downstream of Akt and mTOR, recapitulates Akt's action in tumorigenesis and drug resistance, but is unable to confer sensitivity to rapamycin and chemotherapy. These results establish Akt signalling through mTOR and eIF4E as an important mechanism of oncogenesis and drug resistance in vivo, and reveal how targeting apoptotic programmes can restore drug sensitivity in a genotype-dependent manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000)
Johnstone, R. W., Ruefli, A. A. & Lowe, S. W. Apoptosis: a link between cancer genetics and chemotherapy. Cell 108, 153–164 (2002)
Mayo, L. D., Dixon, J. E., Durden, D. L., Tonks, N. K. & Donner, D. B. PTEN protects p53 from Mdm2 and sensitizes cancer cells to chemotherapy. J. Biol. Chem. 277, 5484–5489 (2002)
Datta, S. R., Brunet, A. & Greenberg, M. E. Cellular survival: a play in three Akts. Genes Dev. 13, 2905–2927 (1999)
Vivanco, I. & Sawyers, C. L. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nature Rev. Cancer 2, 489–501 (2002)
Steck, P. A. et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nature Genet. 15, 356–362 (1997)
Sakai, A., Thieblemont, C., Wellmann, A., Jaffe, E. S. & Raffeld, M. PTEN gene alterations in lymphoid neoplasms. Blood 92, 3410–3415 (1998)
Min, Y. H. et al. Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: its significance as a prognostic variable. Leukemia 17, 995–997 (2003)
Andjelkovic, M. et al. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272, 31515–31524 (1997)
Adams, J. M. et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318, 533–538 (1985)
Schmitt, C. A. et al. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell 1, 289–298 (2002)
Schmitt, C. A., Rosenthal, C. T. & Lowe, S. W. Genetic analysis of chemoresistance in primary murine lymphomas. Nature Med. 6, 1029–1035 (2000)
Schmitt, C. A. et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 109, 335–346 (2002)
Huang, S. & Houghton, P. J. Targeting mTOR signalling for cancer therapy. Curr. Opin. Pharmacol. 3, 371–377 (2003)
Plas, D. R., Talapatra, S., Edinger, A. L., Rathmell, J. C. & Thompson, C. B. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J. Biol. Chem. 276, 12041–12048 (2001)
Edinger, A. L. & Thompson, C. B. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell 13, 2276–2288 (2002)
Neshat, M. S. et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc. Natl Acad. Sci. USA 98, 10314–10319 (2001)
Grunwald, V. et al. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res. 62, 6141–6145 (2002)
Podsypanina, K. et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/- mice. Proc. Natl Acad. Sci. USA 98, 10320–10325 (2001)
Hosoi, H. et al. Rapamycin causes poorly reversible inhibition of mTOR and induces p53-independent apoptosis in human rhabdomyosarcoma cells. Cancer Res. 59, 886–894 (1999)
Schmelzle, T. & Hall, M. N. TOR, a central controller of cell growth. Cell 103, 253–262 (2000)
Lazaris-Karatzas, A., Montine, K. S. & Sonenberg, N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature 345, 544–547 (1990)
Polunovsky, V. A. et al. Translational control of the antiapoptotic function of Ras. J. Biol. Chem. 275, 24776–24780 (2000)
Hershey, J. W. B. & Miyamoto, S. in Translational Control of Gene Expression (eds Sonenberg, N., Hershey, J. W. B. & Mathews, M. B.) 637–654 (Cold Spring Harbor, New York, 2000)
Grolleau, A. et al. Global and specific translational control by rapamycin in T cells uncovered by microarrays and proteomics. J. Biol. Chem. 277, 22175–22184 (2002)
Rajasekhar, V. K. et al. Oncogenic Ras and Akt signalling contribute to glioblastoma formation by differential recruitment of existing mRNAs to polysomes. Mol. Cell 12, 889–901 (2003)
Schmitt, C. A., McCurrach, M. E., de Stanchina, E., Wallace-Brodeur, R. R. & Lowe, S. W. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 13, 2670–2677 (1999)
Yang, M. et al. Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proc. Natl Acad. Sci. USA 97, 1206–1211 (2000)
Di Cristofano, A., De Acetis, M., Koff, A., Cordon-Cardo, C. & Pandolfi, P. P. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nature Genet. 27, 222–224 (2001)
de Stanchina, E. et al. E1A signalling to p53 involves the p19(ARF) tumor suppressor. Genes Dev. 12, 2434–2442 (1998)
Acknowledgements
We thank M. Myers and N. Sonenberg for reagents; C. Rosenthal, M. S. Jiao, P. Chan, M. L. Maunakea and F. Baehner for technical assistance; and L. Bianco for guidance on animal studies. We also thank C. Thompson and members of the Lowe laboratory for discussions, and M. McCurrach, M. Hemann, E. Cepero and D. Burgess for editorial advice. This work was supported by a gift from the Ann L. and Herbert J. Siegel Philanthropic Fund and the Laurie Strauss Leukaemia Foundation, an AACR/Amgen Fellowship in Translational Research (H.-G.W.), a Tularik Post-doctoral Fellowship (E.d.S), a NSERC graduate scholarship (A.M.), an NCI postdoctoral training grant (J.S.F), grants from Canadian Institutes of Health Research and National Cancer Institute of Canada (J.P.), the Mouse Models of Human Cancer Consortium and a Burroughs Wellcome Fund Career Award (S.K.), a SCOR grant from the Leukaemia and Lymphoma Society (S.K and S.W.L.), and a program project grant from the National Cancer Institute (S.W.L and C.C.-C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
Representative data from flow cytometric immunophenotyping of control (myc), Bcl-2 (myc/bcl-2), Akt (myc/akt) and eIF4E (myc/eIF4E) tumours. (PDF 283 kb)
Supplementary Figure 2
Allele-specific PCR to detect the wild-type (p53 WT) and mutant allele (p53 Neo) in tumours derived from Eµ-myc/p53+/- HSCs. (PDF 51 kb)
Supplementary Figure 3
Kaplan-Meier plots detailing the survival time following treatment with CTX (a) and DXR (b). (PDF 7 kb)
Supplementary Figure 4
Overall survival of mice treated with rapamycin alone or in combination with conventional chemotherapy. (PDF 11 kb)
Supplementary Figure 5
Kaplan-Meier analysis of tumour free survival in Akt tumour bearing mice (a) following treatment with CTX (n = 16, red), RAP (n = 12, blue), or CTX+RAP (C+R, n = 8) and in Bcl-2 tumour bearing mice (b) treated with CTX (n = 6, red), RAP (n = 6, blue) and CTX+RAP (C+R, n=4, green). (PDF 8 kb)
Supplementary Figure 6
Rapamycin reverses chemoresistance in matched Akt-expressing lymphomas. (PDF 4 kb)
Supplementary Figure 7
Quantification of eIF4E expression in lysates derived from Bcl-2 (n=3), Akt (n=5) and eIF4E (n=5) tumours. (PDF 48 kb)
Supplementary Table 1
Immunophenotype of Emmyc tumours expressing Akt, Bcl-2 or eIF4E. PDF, 168kB (PDF 164 kb)
Rights and permissions
About this article
Cite this article
Wendel, HG., Stanchina, E., Fridman, J. et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature 428, 332–337 (2004). https://doi.org/10.1038/nature02369
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02369
This article is cited by
-
Bromodomain and extraterminal (BET) proteins: biological functions, diseases, and targeted therapy
Signal Transduction and Targeted Therapy (2023)
-
Liquid biopsy in ovarian cancer: advantages and limitations for prognosis and diagnosis
Medical Oncology (2023)
-
Small biomarkers with massive impacts: PI3K/AKT/mTOR signalling and microRNA crosstalk regulate nasopharyngeal carcinoma
Biomarker Research (2022)
-
FAM126A interacted with ENO1 mediates proliferation and metastasis in pancreatic cancer via PI3K/AKT signaling pathway
Cell Death Discovery (2022)
-
Aspirin sensitivity of PIK3CA-mutated Colorectal Cancer: potential mechanisms revisited
Cellular and Molecular Life Sciences (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.