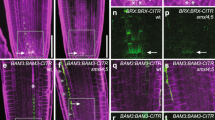
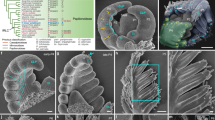
Abstract
In both animals and plants, many developmentally important regulatory genes have complementary microRNAs (miRNAs), which suggests that these miRNAs constitute a class of developmental signalling molecules1. Leaves of higher plants exhibit a varying degree of asymmetry along the adaxial/abaxial (upper/lower) axis. This asymmetry is specified through the polarized expression of class III homeodomain/leucine zipper (HD-ZIPIII) genes2,3,4. In Arabidopsis, three such genes, PHABULOSA (PHB), PHAVOLUTA (PHV) and REVOLUTA (REV), are expressed throughout the incipient leaf, but become adaxially localized after primordium emergence. Downregulation of the HD-ZIPIII genes allows expression of the KANADI and YABBY genes, which specify abaxial fate5,6,7,8. PHB, PHV and REV transcripts contain a complementary site for miRNA165 and miRNA166, which can direct their cleavage in vitro9,10,11. Here we show that miRNA166 constitutes a highly conserved polarizing signal whose expression pattern spatially defines the expression domain of the maize hd-zipIII family member rolled leaf1 (rld1). Moreover, the progressively expanding expression pattern of miRNA166 during leaf development and its accumulation in phloem suggests that miRNA166 may form a movable signal that emanates from a signalling centre below the incipient leaf.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Carrington, J. C. & Ambros, V. Role of microRNAs in plant and animal development. Science 301, 336–338 (2003)
McConnell, J. R. et al. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411, 709–713 (2001)
Emery, J. F. et al. Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr. Biol. 13, 1768–1774 (2003)
Otsuga, D., DeGuzman, B., Prigge, M. J., Drews, G. N. & Clark, S. E. REVOLUTA regulates meristem initiation at lateral positions. Plant J. 25, 223–236 (2001)
Eshed, Y., Baum, S. F., Perea, J. V. & Bowman, J. L. Establishment of polarity in lateral organs of plants. Curr. Biol. 11, 1251–1260 (2001)
Kerstetter, R. A., Bollman, K., Taylor, R. A., Bomblies, K. & Poethig, R. S. KANADI regulates organ polarity in Arabidopsis. Nature 411, 706–709 (2001)
Sawa, S. et al. FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes Dev. 13, 1079–1088 (1999)
Siegfried, K. R. et al. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development 126, 4117–4128 (1999)
Reinhart, B. J., Weinstein, E. G., Rhoades, M. W., Bartel, B. & Bartel, D. P. MicroRNAs in plants. Genes Dev. 16, 1616–1626 (2002)
Rhoades, M. W. et al. Prediction of plant microRNA targets. Cell 110, 513–520 (2002)
Tang, G., Reinhart, B. J., Bartel, D. P. & Zamore, P. D. A biochemical framework for RNA silencing in plants. Genes Dev. 17, 49–63 (2003)
McConnell, J. R. & Barton, M. K. Leaf polarity and meristem formation in Arabidopsis. Development 125, 2935–2942 (1998)
Nelson, J. M., Lane, B. & Freeling, M. Expression of a mutant maize gene in the ventral leaf epidermis is sufficient to signal a switch of the leaf's dorsoventral axis. Development 129, 4581–4589 (2002)
Llave, C., Kasschau, K. D., Rector, M. A. & Carrington, J. C. Endogenous and silencing-associated small RNAs in plants. Plant Cell 14, 1605–1619 (2002)
Park, W., Li, J., Song, R., Messing, J. & Chen, X. CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr. Biol. 12, 1484–1495 (2002)
Aukerman, M. J. & Sakai, H. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15, 2730–2741 (2003)
Palatnik, J. F. et al. Control of leaf morphogenesis by microRNAs. Nature 425, 257–263 (2003)
Zhong, R. & Ye, Z. H. IFL1, a gene regulating interfascicular fiber differentiation in Arabidopsis, encodes a homeodomain-leucine zipper protein. Plant Cell 11, 2139–2152 (1999)
Ratcliffe, O. J., Riechmann, J. L. & Zhang, J. Z. INTERFASCICULAR FIBERLESS1 is the same gene as REVOLUTA. Plant Cell 12, 315–317 (2000)
Llave, C., Xie, Z., Kasschau, K. D. & Carrington, J. C. Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 297, 2053–2056 (2002)
Timmermans, M. C. P., Schultes, N. P., Jankovsky, J. P. & Nelson, T. Leafbladeless1 is required for dorsoventrality of lateral organs in maize. Development 125, 2813–2823 (1998)
Sussex, I. M. Morphogenesis in Solanum tuberosum L.: Experimental investigation of leaf dorsiventrality and orientation in the juvenile shoot. Phytomorphology 5, 286–300 (1955)
Snow, M. & Snow, R. The dorsiventrality of leaf primordia. New Phytol. 58, 188–207 (1959)
Foster, T. M. et al. A surveillance system regulates selective entry of RNA into the shoot apex. Plant Cell 14, 1479–1508 (2002)
Burr, B., Burr, F. A., Thompson, K. H., Albertson, M. C. & Stuber, C. W. Gene mapping with recombinant inbreds in maize. Genetics 118, 519–526 (1988)
Timmermans, M. C. P., Das, O. P. & Messing, J. Characterization of a meiotic crossover in maize identified by a restriction fragment length polymorphism-based method. Genetics 143, 1771–1783 (1996)
Jackson, D. in Molecular Plant Pathology: A Practical Approach (eds Bowles, D. J., Gurr, S. J. & McPherson, M.) 163–174 (Oxford Univ. Press, Oxford, 1991)
Acknowledgements
We thank T. Phelps-Durr, C. Kidner, T. Volpe and G. Hannon for discussions and comments on the manuscript. We also thank T. Mulligan for plant care. This work was supported by grants from the USDA. and the NSF (to M.C.P.T.). M.T.J. is funded in part by a W. Burghardt Turner Fellowship, and J.S.K. is an Alfred Hershey Fellow of the Watson School of Biological Sciences and is supported by a grant from the National Institute of General Medical Sciences, NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Juarez, M., Kui, J., Thomas, J. et al. microRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature 428, 84–88 (2004). https://doi.org/10.1038/nature02363
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02363
This article is cited by
-
Whole transcriptome analysis and construction of a ceRNA regulatory network related to leaf and petiole development in Chinese cabbage (Brassica campestris L. ssp. pekinensis)
BMC Genomics (2023)
-
A potential candidate gene associated with the angles of the ear leaf and the second leaf above the ear leaf in maize
BMC Plant Biology (2023)
-
Auxin efflux carrier ZmPIN1a modulates auxin reallocation involved in nitrate-mediated root formation
BMC Plant Biology (2023)
-
RWP-RK Domain 3 (OsRKD3) induces somatic embryogenesis in black rice
BMC Plant Biology (2023)
-
A Wox3-patterning module organizes planar growth in grass leaves and ligules
Nature Plants (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.