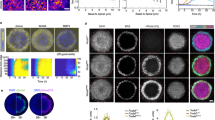
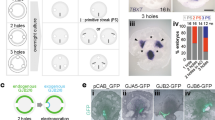
Abstract
During vertebrate embryo development, the breaking of the initial bilateral symmetry is translated into asymmetric gene expression around the node and/or in the lateral plate mesoderm. The earliest conserved feature of this asymmetric gene expression cascade is the left-sided expression of Nodal, which depends on the activity of the Notch signalling pathway. Here we present a mathematical model describing the dynamics of the Notch signalling pathway during chick embryo gastrulation, which reveals a complex and highly robust genetic network that locally activates Notch on the left side of Hensen's node. We identify the source of the asymmetric activation of Notch as a transient accumulation of extracellular calcium, which in turn depends on left–right differences in H+/K+-ATPase activity. Our results uncover a mechanism by which the Notch signalling pathway translates asymmetry in epigenetic factors into asymmetric gene expression around the node.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Burdine, R. D. & Schier, A. F. Conserved and divergent mechanisms in left–right axis formation. Genes Dev. 14, 763–776 (2000)
Capdevila, J., Vogan, K. J., Tabin, C. J. & Izpisúa Belmonte, J. C. Mechanisms of left–right determination in vertebrates. Cell 101, 9–21 (2000)
Mercola, M. & Levin, M. Left–right asymmetry determination in vertebrates. Annu. Rev. Cell Dev. Biol. 17, 779–805 (2001)
Hamada, H., Meno, C., Watanabe, D. & Saijoh, Y. Establishment of vertebrate left–right asymmetry. Nature Rev. Genet. 3, 103–113 (2002)
Bisgrove, B. W., Morelli, S. H. & Yost, H. J. Genetics of human laterality disorders: insights from vertebrate model systems. Annu. Rev. Genomics Hum. Genet. 4, 1–32 (2003)
Brennan, J., Norris, D. P. & Robertson, E. J. Nodal activity in the node governs left–right asymmetry. Genes Dev. 16, 2339–2344 (2002)
Saijoh, Y., Oki, S., Ohishi, S. & Hamada, H. Left–right patterning of the mouse lateral plate requires nodal produced in the node. Dev. Biol. 256, 160–172 (2003)
Krebs, L. T. et al. Notch signaling regulates left–right asymmetry determination by inducing Nodal expression. Genes Dev. 17, 1207–1212 (2003)
Raya, A. et al. Notch activity induces Nodal expression and mediates the establishment of left–right asymmetry in vertebrate embryos. Genes Dev. 17, 1213–1218 (2003)
Artavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 (1999)
Hamburger, V. & Hamilton, H. L. A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49–92 (1951)
Jouve, C., Iimura, T. & Pourquie, O. Onset of the segmentation clock in the chick embryo: evidence for oscillations in the somite precursors in the primitive streak. Development 129, 1107–1117 (2002)
Irvine, K. D. Fringe, Notch, and making developmental boundaries. Curr. Opin. Genet. Dev. 9, 434–441 (1999)
Evrard, Y. A., Lun, Y., Aulehla, A., Gan, L. & Johnson, R. L. lunatic fringe is an essential mediator of somite segmentation and patterning. Nature 394, 377–381 (1998)
Zhang, N. & Gridley, T. Defects in somite formation in lunatic fringe-deficient mice. Nature 394, 374–377 (1998)
Dale, J. K. et al. Periodic notch inhibition by lunatic fringe underlies the chick segmentation clock. Nature 421, 275–278 (2003)
Geling, A., Steiner, H., Willem, M., Bally-Cuif, L. & Haass, C. A γ-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 3, 688–694 (2002)
Gibson, G. Developmental evolution: getting robust about robustness. Curr. Biol. 12, R347–R349 (2002)
Stern, C. D. et al. Activin and its receptors during gastrulation and the later phases of mesoderm development in the chick embryo. Dev. Biol. 172, 192–205 (1995)
Levin, M., Johnson, R. L., Stern, C. D., Kuehn, M. & Tabin, C. A molecular pathway determining left–right asymmetry in chick embryogenesis. Cell 82, 803–814 (1995)
Boettger, T., Wittler, L. & Kessel, M. FGF8 functions in the specification of the right body side of the chick. Curr. Biol. 9, 277–280 (1999)
Hirokawa, N. Stirring up development with the heterotrimeric kinesin KIF3. Traffic 1, 29–34 (2000)
Wagner, M. K. & Yost, H. J. Left–right development: the roles of nodal cilia. Curr. Biol. 10, R149–R151 (2000)
Levin, M., Thorlin, T., Robinson, K., Nogi, T. & Mercola, M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left–right patterning. Cell 111, 77–89 (2002)
Stern, C. D. & Wolpert, L. Left–right asymmetry: all hands to the pump. Curr. Biol. 12, R802–R803 (2002)
Mercola, M. Left–right asymmetry: nodal points. J. Cell Sci. 116, 3251–3257 (2003)
Robinson, K. R. & Messerli, M. A. Left/right, up/down: The role of endogenous electrical fields as directional signals in development, repair and invasion. Bioessays 25, 759–766 (2003)
Rao, Z. et al. The structure of a Ca2+-binding epidermal growth factor-like domain: its role in protein-protein interactions. Cell 82, 131–141 (1995)
Rand, M. D., Lindblom, A., Carlson, J., Villoutreix, B. O. & Stenflo, J. Calcium binding to tandem repeats of EGF-like modules. Expression and characterization of the EGF-like modules of human Notch-1 implicated in receptor–ligand interactions. Protein Sci. 6, 2059–2071 (1997)
McGrath, J., Somlo, S., Makova, S., Tian, X. & Brueckner, M. Two populations of node monocilia initiate left–right asymmetry in the mouse. Cell 114, 61–73 (2003)
Hicks, C. et al. Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nature Cell Biol. 2, 515–520 (2000)
Jarriault, S. et al. Signalling downstream of activated mammalian Notch. Nature 377, 355–358 (1995)
Rand, M. D. et al. Calcium depletion dissociates and activates heterodimeric notch receptors. Mol. Cell. Biol. 20, 1825–1835 (2000)
Jaffe, L. F. Organization of early development by calcium patterns. Bioessays 21, 657–667 (1999)
New, D. A. T. A new technique for the cultivation of the chick embryo in vitro. J. Embryol. Exp. Morphol. 3, 326–331 (1955)
Ryan, A. K. et al. Pitx2 determines left–right asymmetry of internal organs in vertebrates. Nature 394, 545–551 (1998)
Itasaki, N., Bel-Vialar, S. & Krumlauf, R. ‘Shocking’ developments in chick embryology: electroporation and in ovo gene expression. Nature Cell Biol. 1, E203–E207 (1999)
Izpisúa Belmonte, J. C., De Robertis, E. M., Storey, K. G. & Stern, C. D. The homeobox gene goosecoid and the origin of organizer cells in the early chick blastoderm. Cell 74, 645–659 (1993)
Rodríguez-Esteban, C. et al. The novel Cer-like protein Caronte mediates the establishment of embryonic left–right asymmetry. Nature 401, 243–251 (1999)
Laufer, E., Nelson, C. E., Johnson, R. L., Morgan, B. A. & Tabin, C. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell 79, 993–1003 (1994)
Talora, C., Sgroi, D. C., Crum, C. P. & Dotto, G. P. Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes Dev. 16, 2252–2263 (2002)
Lindsell, C. E., Shawber, C. J., Boulter, J. & Weinmaster, G. Jagged: a mammalian ligand that activates Notch1. Cell 80, 909–917 (1995)
Shi, S. & Stanley, P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc. Natl Acad. Sci. USA 100, 5234–5239 (2003)
Panin, V. M., Papayannopoulos, V., Wilson, R. & Irvine, K. D. Fringe modulates Notch–ligand interactions. Nature 387, 908–912 (1997)
Bruckner, K., Perez, L., Clausen, H. & Cohen, S. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature 406, 411–415 (2000)
Moloney, D. J. et al. Fringe is a glycosyltransferase that modifies Notch. Nature 406, 369–375 (2000)
Murray, J. D. Mathematical Biology (Springer, Berlin, 1993)
Bosenberg, M. W. & Massague, J. Juxtacrine cell signaling molecules. Curr. Opin. Cell Biol. 5, 832–838 (1993)
Collier, J. R., Monk, N. A., Maini, P. K. & Lewis, J. H. Pattern formation by lateral inhibition with feedback: a mathematical model of delta-notch intercellular signalling. J. Theor. Biol. 183, 429–446 (1996)
Owen, M. R., Sherratt, J. A. & Wearing, H. J. Lateral induction by juxtacrine signaling is a new mechanism for pattern formation. Dev. Biol. 217, 54–61 (2000)
Acknowledgements
We thank M-F. Schwarz and H. Pineda for their technical skills and dedication; A. Lehrman and H. Juguilon for help with the luciferase assays; G. Sternik for assistance in two-photon excitation microscopy analysis; all laboratory members for discussions; L. Hooks for help in preparing the manuscript; C. Fryers, K. Jones and C. Kintner for reagents and discussions; and T. Gridley and R. Johnson for sharing unpublished results. A.R. is partially supported by a postdoctoral fellowship from the Ministerio de Educación, Cultura y Deporte, Spain; J.R.L is supported by a fellowship from Fundação para a Ciencia e a Tecnologia, Portugal, and M.I. is partially supported by the Fulbright Program and Generalitat of Catalunya. This work was supported by grants from the Fundação Calouste Gulbenkian e Fundação para a Ciencia e a Tecnologia, the American Heart Association, the Human Frontier Science Program, the NIH and the G. Harold and Leila Y. Mathers Charitable Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Raya, Á., Kawakami, Y., Rodríguez-Esteban, C. et al. Notch activity acts as a sensor for extracellular calcium during vertebrate left–right determination. Nature 427, 121–128 (2004). https://doi.org/10.1038/nature02190
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02190
This article is cited by
-
Regulatory changes associated with the head to trunk developmental transition
BMC Biology (2023)
-
Light-induced asymmetries in embryonic retinal gene expression are mediated by the vascular system and extracellular matrix
Scientific Reports (2022)
-
Notch signaling pathway in infectious diseases: role in the regulation of immune response
Inflammation Research (2021)
-
The relationship between biological function and teleology: Implications for biology education
Evolution: Education and Outreach (2020)
-
Sustained Depolarization of the Resting Membrane Potential Regulates Muscle Progenitor Cell Growth and Maintains Stem Cell Properties In Vitro
Stem Cell Reviews and Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.