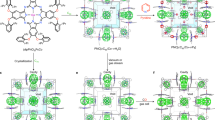
Abstract
Natural porous solids such as zeolites are invariably formed with inorganic cations such as Na+ and K+ (refs 1, 2). However, current research on new porous materials is mainly focused on the use of organic species as either structure-directing or structure-building units; purely inorganic systems have received relatively little attention in exploratory synthetic work3,4,5,6,7,8,9. Here we report the synthesis of a series of three-dimensional sulphides and selenides containing highly mobile alkali metal cations as charge-balancing extra-framework cations. Such crystalline inorganic chalcogenides integrate zeolite-like architecture with high anionic framework polarizability and high concentrations of mobile cations. Such structural features are particularly desirable for the development of fast-ion conductors10. These materials demonstrate high ionic conductivity (up to 1.8 × 10-2 ohm-1 cm-1) at room temperature and moderate to high humidity. This synthetic methodology, together with novel structural, physical and chemical properties, may lead to the development of new microporous and open-framework materials with potential applications in areas such as batteries, fuel cells, electrochemical sensors and photocatalysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Flanigen, E. M. in Introduction to Zeolite Science and Practice (eds van Bekkum, H., Flanigen, E. M. & Jansen, J. C.) 13–34 (Elsevier, New York, 1991)
Breck, D. W. Zeolite Molecular Sieves, Structure, Chemistry, and Use (John Wiley & Sons, New York, 1974)
Davis, M. E. Ordered porous materials for emerging applications. Nature 417, 813 (2002)
Feng, P., Bu, X. & Stucky, G. D. Hydrothermal syntheses and structural characterization of zeolite analogue compounds based on cobalt phosphate. Nature 388, 735–741 (1997)
Scott, R. W. J., MacLachlan, M. J. & Ozin, G. A. Synthesis of metal sulfide materials with controlled architecture. Curr. Opin. Solid State Mater. Sci. 4, 113–121 (1999)
Huo, Q., Leon, R., Petroff, P. M. & Stucky, G. D. Mesostructure design with gemini surfactants: supercage formation in a three-dimensional hexagonal array. Science 68, 1324–1327 (1995)
Zhao, D. et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279, 548–552 (1998)
Johnson, S. A., Ollivier, P. J. & Mallouk, T. E. Ordered mesoporous polymers of tunable pore size from colloidal silica templates. Science 283, 963–965 (1999)
Cheetham, A. K., Ferey, G. & Loiseau, T. Open-framework inorganic materials. Angew. Chem. Int. Edn 38, 3268–3292 (1999)
West, A. R. Solid State Chemistry and its Applications (Wiley, New York, 1992)
Zheng, N., Bu, X. & Feng, P. Microporous and photoluminescent chalcogenide zeolite analogs. Science 298, 2366–2369 (2002)
Bedard, R. L., Wilson, S. T., Vail, L. D., Bennett, J. M. & Flanigen, E. M. in Zeolites: Facts, Figures, Future. Proc. 8th Int. Zeolite Conf. (eds Jacobs, P. A. & van Santen, R. A.) 375 (Elsevier, Amsterdam, 1989)
Cahill, C. L. & Parise, J. B. On the formation of framework indium sulfides. J. Chem. Soc. Dalton Trans. 1475–1482 (2000)
Dhingra, S. & Kanatzidis, M. G. Open framework structures based on Sex2- fragments: synthesis of (Ph4P)[M(Se6)2] (M = Ga, In, Tl) in molten (Ph4P)2Sex . Science 258, 1769–1772 (1992)
Li, H., Laine, A., O'Keeffe, M. & Yaghi, O. M. Supertetrahedral sulfide crystals with giant cavities and channels. Science 283, 1145–1147 (1999)
Wehmschulte, R. J. & Power, P. P. Low-temperature synthesis of aluminum sulfide as the solvate Al4S6(NMe3)4 in hydrocarbon solution. J. Am. Chem. Soc. 119, 9566–9567 (1997)
Bu, X., Zheng, N., Li, Y. & Feng, P. Pushing up the size limit of chalcogenide supertetrahedral clusters: two- and three-dimensional photoluminescent open frameworks from (Cu5In30S54)13- clusters. J. Am. Chem. Soc. 124, 12646–12647 (2002)
Hoppe, R., Lidecke, W. & Frotath, F. C. Sodium thioindate and sodium selenoindate. Z. Anorg. Allgem. Chem. 309, 49–54 (1961)
Acknowledgements
We acknowledge the support of this work by the NSF. We also thank Y. Yan and his group for assistance with impedance measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Zheng, N., Bu, X. & Feng, P. Synthetic design of crystalline inorganic chalcogenides exhibiting fast-ion conductivity. Nature 426, 428–432 (2003). https://doi.org/10.1038/nature02159
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02159
This article is cited by
-
Interface engineering for enhancing the performance of novel sodium-doped MoS2 nanocomposite: Synthesis and characterization functioning as a high-performance supercapacitor
Korean Journal of Chemical Engineering (2023)
-
A chalcogenide-cluster-based semiconducting nanotube array with oriented photoconductive behavior
Nature Communications (2021)
-
MoS2-based nanocomposites: synthesis, structure, and applications in water remediation and energy storage: a review
Environmental Chemistry Letters (2021)
-
Probing the Electrochemical Properties of Flower Like Mesoporous MoS2 in Different Aqueous Electrolytes
Journal of Electronic Materials (2019)
-
All-Solid-State Supercapacitor Based on MoS2–Graphite Composite Prepared by the Vacuum Kinetic Spray Method
Journal of Thermal Spray Technology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.