Abstract
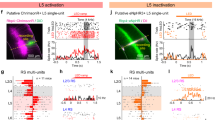
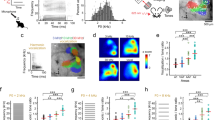
Neurons in the primary auditory cortex are tuned to the intensity and specific frequencies of sounds, but the synaptic mechanisms underlying this tuning remain uncertain. Inhibition seems to have a functional role in the formation of cortical receptive fields, because stimuli often suppress similar or neighbouring responses1,2,3, and pharmacological blockade of inhibition broadens tuning curves4,5. Here we use whole-cell recordings in vivo to disentangle the roles of excitatory and inhibitory activity in the tone-evoked responses of single neurons in the auditory cortex. The excitatory and inhibitory receptive fields cover almost exactly the same areas, in contrast to the predictions of classical lateral inhibition models. Thus, although inhibition is typically as strong as excitation, it is not necessary to establish tuning, even in the receptive field surround. However, inhibition and excitation occurred in a precise and stereotyped temporal sequence: an initial barrage of excitatory input was rapidly quenched by inhibition, truncating the spiking response within a few (1–4) milliseconds. Balanced inhibition might thus serve to increase the temporal precision6 and thereby reduce the randomness of cortical operation, rather than to increase noise as has been proposed previously7.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Shamma, S. A. & Symmes, D. Patterns of inhibition in auditory cortical cells in awake squirrel monkeys. Hear. Res. 19, 1–13 (1985)
Calford, M. B. & Semple, M. N. Monaural inhibition in cat auditory cortex. J. Neurophysiol. 73, 1876–1891 (1995)
Sutter, M. L., Schreiner, C. E., McLean, M., O'Connor, K. N. & Loftus, W. C. Organization of inhibitory frequency receptive fields in cat primary auditory cortex. J. Neurophysiol. 82, 2358–2371 (1999)
Chen, Q. C. & Jen, P. H. Bicuculline application affects discharge patterns, rate-intensity functions, and frequency tuning characteristics of bat auditory cortical neurons. Hear. Res. 150, 161–174 (2000)
Wang, J., McFadden, S. L., Caspary, D. & Salvi, R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res. 944, 219–231 (2002)
Pouille, F. & Scanziani, M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293, 1159–1163 (2001)
Shadlen, M. N. & Newsome, W. T. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J. Neurosci. 18, 3870–3896 (1998)
Hartline, H. K., Wagner, H. G. & Ratcliff, F. Inhibition in the eye of Limulus. J. Gen. Physiol. 39, 651–673 (1956)
Zhang, L. I., Tan, A. Y., Schreiner, C. E. & Merzenich, M. M. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature 424, 201–205 (2003)
Borg-Graham, L. J., Monier, C. & Fregnac, Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature 393, 369–373 (1998)
Hirsch, J. A., Alonso, J. M., Reid, R. C. & Martinez, L. M. Synaptic integration in striate cortical simple cells. J. Neurosci. 18, 9517–9528 (1998)
Anderson, J. S., Carandini, M. & Ferster, D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J. Neurophysiol. 84, 909–926 (2000)
Monier, C., Chavane, F., Baudot, P., Graham, L. J. & Fregnac, Y. Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron 37, 663–680 (2003)
Moore, C. I. & Nelson, S. B. Spatio-temporal subthreshold receptive fields in the vibrissa representation of rat primary somatosensory cortex. J. Neurophysiol. 80, 2882–2892 (1998)
Carandini, M. & Ferster, D. Membrane potential and firing rate in cat primary visual cortex. J. Neurosci. 20, 470–484 (2000)
Ojima, H. & Murakami, K. Intracellular characterization of suppressive responses in supragranular pyramidal neurons of cat primary auditory cortex in vivo. Cereb. Cortex 12, 1079–1091 (2002)
Chung, S. & Ferster, D. Strength and orientation tuning of the thalamic input to simple cells revealed by electrically evoked cortical suppression. Neuron 20, 1177–1189 (1998)
Miller, L. M., Escabi, M. A., Read, H. L. & Schreiner, C. E. Functional convergence of response properties in the auditory thalamocortical system. Neuron 32, 151–160 (2001)
Schummers, J., Marino, J. & Sur, M. Synaptic integration by V1 neurons depends on location within the orientation map. Neuron 36, 969–978 (2002)
Phillips, D. P. & Cynader, M. S. Some neural mechanisms in the cat's auditory cortex underlying sensitivity to combined tone and wide-spectrum noise stimuli. Hear. Res. 18, 87–102 (1985)
Volkov, I. O. & Galazyuk, A. V. Peculiarities of inhibition in cat auditory cortex neurons evoked by tonal stimuli of various durations. Exp. Brain Res. 91, 115–120 (1992)
Chung, S., Li, X. & Nelson, S. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron 34, 437–446 (2002)
Chance, F. S., Abbott, L. F. & Reyes, A. D. Gain modulation from background synaptic input. Neuron 35, 773–782 (2002)
Phillips, D. P. Effect of tone-pulse rise time on rate-level functions of cat auditory cortex neurons: excitatory and inhibitory processes shaping responses to tone onset. J. Neurophysiol. 59, 1524–1539 (1988)
De Ribaupierre, F., Goldstein, M. H. Jr & Yeni-Komshian, G. Intracellular study of the cat's primary auditory cortex. Brain Res. 48, 185–204 (1972)
Mitani, A. & Shimokouchi, M. Neuronal connections in the primary auditory cortex: an electrophysiological study in the cat. J. Comp. Neurol. 235, 417–429 (1985)
Brugge, J. F., Dubrovsky, N. A., Aitkin, L. M. & Anderson, D. J. Sensitivity of single neurons in auditory cortex of cat to binaural tonal stimulation; effects of varying interaural time and intensity. J. Neurophysiol. 32, 1005–1024 (1969)
DeWeese, M. R., Wehr, M. & Zador, A. M. Binary spiking in auditory cortex. J. Neurosci. 23, 7940–7949 (2003)
Covey, E., Kauer, J. A. & Casseday, J. H. Whole-cell patch-clamp recording reveals subthreshold sound-evoked postsynaptic currents in the inferior colliculus of awake bats. J. Neurosci. 16, 3009–3018 (1996)
Stevens, C. F. & Zador, A. M. Input synchrony and the irregular firing of cortical neurons. Nature Neurosci. 1, 210–217 (1998)
Acknowledgements
We thank K. Miller, L. Miller, M. Kvale, Z. Mainen, M. DeWeese and M. Sutter for comments on an earlier version of this manuscript. This work has been supported by grants to A.M.Z. from the Packard Foundation, the Sloan Foundation, the NIH and the Mathers Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Wehr, M., Zador, A. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426, 442–446 (2003). https://doi.org/10.1038/nature02116
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02116
This article is cited by
-
Local origin of excitatory–inhibitory tuning equivalence in a cortical network
Nature Neuroscience (2024)
-
On the physiological and structural contributors to the overall balance of excitation and inhibition in local cortical networks
Journal of Computational Neuroscience (2024)
-
Cell-type-specific plasticity of inhibitory interneurons in the rehabilitation of auditory cortex after peripheral damage
Nature Communications (2023)
-
Parvalbumin neurons enhance temporal coding and reduce cortical noise in complex auditory scenes
Communications Biology (2023)
-
Developmental loss of ErbB4 in PV interneurons disrupts state-dependent cortical circuit dynamics
Molecular Psychiatry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.