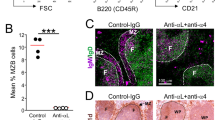
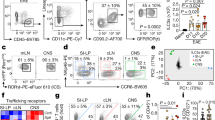
Abstract
Peripheral infection is the natural route of transmission in most prion diseases1. Peripheral prion infection is followed by rapid prion replication in lymphoid organs, neuroinvasion2 and progressive neurological disease. Both immune cells and nerves are involved in pathogenesis3,4, but the mechanisms of prion transfer from the immune to the nervous system are unknown. Here we show that ablation of the chemokine receptor CXCR5 juxtaposes follicular dendritic cells (FDCs) to major splenic nerves, and accelerates the transfer of intraperitoneally administered prions into the spinal cord. Neuroinvasion velocity correlated exclusively with the relative locations of FDCs and nerves: transfer of CXCR5-/- bone marrow to wild-type mice induced perineural FDCs and enhanced neuroinvasion, whereas reciprocal transfer to CXCR5-/- mice abolished them and restored normal efficiency of neuroinvasion. Suppression of lymphotoxin signalling depleted FDCs, abolished splenic infectivity, and suppressed acceleration of pathogenesis in CXCR5-/- mice. This suggests that prion neuroimmune transition occurs between FDCs and sympathetic nerves, and relative positioning of FDCs and nerves controls the efficiency of peripheral prion infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Aguzzi, A., Montrasio, F. & Kaeser, P. S. Prions: health scare and biological challenge. Nature Rev. Mol. Cell Biol. 2, 118–126 (2001)
Aguzzi, A. Neuro-immune connection in spread of prions in the body? Lancet 349, 742–743 (1997)
Klein, M. A. et al. A crucial role for B cells in neuroinvasive scrapie. Nature 390, 687–690 (1997)
Race, R., Oldstone, M. & Chesebro, B. Entry versus blockade of brain infection following oral or intraperitoneal scrapie administration: role of prion protein expression in peripheral nerves and spleen. J. Virol. 74, 828–833 (2000)
Eklund, C. M., Kennedy, R. C. & Hadlow, W. J. Pathogenesis of scrapie virus infection in the mouse. J. Infect. Dis. 117, 15–22 (1967)
Kimberlin, R. H. & Walker, C. A. The role of the spleen in the neuroinvasion of scrapie in mice. Virus Res. 12, 201–211 (1989)
Prinz, M. et al. Lymph nodal prion replication and neuroinvasion in mice devoid of follicular dendritic cells. Proc. Natl Acad. Sci. USA 99, 919–924 (2002)
Klein, M. A. et al. PrP expression in B lymphocytes is not required for prion neuroinvasion. Nature Med. 4, 1429–1433 (1998)
Montrasio, F. et al. Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science 288, 1257–1259 (2000)
Kitamoto, T., Muramoto, T., Mohri, S., Doh-ura, K. & Tateishi, J. Abnormal isoform of prion protein accumulates in follicular dendritic cells in mice with Creutzfeldt-Jakob disease. J. Virol. 65, 6292–6295 (1991)
Glatzel, M., Heppner, F. L., Albers, K. M. & Aguzzi, A. Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron 31, 25–34 (2001)
Heinen, E., Bosseloir, A. & Bouzahzah, F. Follicular dendritic cells: origin and function. Curr. Top. Microbiol. Immunol. 201, 15–47 (1995)
Forster, R. et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87, 1037–1047 (1996)
Voigt, I. et al. CXCR5-deficient mice develop functional germinal centers in the splenic T cell zone. Eur. J. Immunol. 30, 560–567 (2000)
Felten, D. L. & Felten, S. Y. Sympathetic noradrenergic innervation of immune organs. Brain Behav. Immun. 2, 293–300 (1988)
Carlson, S. L. et al. NGF modulates sympathetic innervation of lymphoid tissues. J. Neurosci. 15, 5892–5899 (1995)
Prusiner, S. B., Cochran, S. P., Downey, D. E. & Groth, D. F. Determination of scrapie agent titer from incubation period measurements in hamsters. Adv. Exp. Med. Biol. 134, 385–399 (1981)
Kaeser, P. S., Klein, M. A., Schwarz, P. & Aguzzi, A. Efficient lymphoreticular prion propagation requires prp(c) in stromal and hematopoietic cells. J. Virol. 75, 7097–7106 (2001)
Fischer, M. et al. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15, 1255–1264 (1996)
Beekes, M., Baldauf, E. & Diringer, H. Sequential appearance and accumulation of pathognomonic markers in the central nervous system of hamsters orally infected with scrapie. J. Gen. Virol. 77, 1925–1934 (1996)
Oldstone, M. B. et al. Lymphotoxin-α- and lymphotoxin-β-deficient mice differ in susceptibility to scrapie: evidence against dendritic cell involvement in neuroinvasion. J. Virol. 76, 4357–4363 (2002)
Mackay, F. & Browning, J. L. Turning off follicular dendritic cells. Nature 395, 26–27 (1998)
Ansel, K. M. et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406, 309–314 (2000)
Hill, A. F., Zeidler, M., Ironside, J. & Collinge, J. Diagnosis of new variant Creutzfeldt-Jakob disease by tonsil biopsy. Lancet 349, 99–100 (1997)
Hilton, D. A., Fathers, E., Edwards, P., Ironside, J. W. & Zajicek, J. Prion immunoreactivity in appendix before clinical onset of variant Creutzfeldt-Jakob disease. Lancet 352, 703–704 (1998)
Aguzzi, A., Glatzel, M., Montrasio, F., Prinz, M. & Heppner, F. L. Interventional strategies against prion diseases. Nature Rev. Neurosci. 2, 745–749 (2001)
Hill, A. F. et al. Species-barrier-independent prion replication in apparently resistant species. Proc. Natl Acad. Sci. USA 29, 10248–10253 (2000)
Karrer, U. et al. Antiviral B cell memory in the absence of mature follicular dendritic cell networks and classical germinal centers in TNFR1 - / - mice. J. Immunol. 164, 768–778 (2000)
Wu, Q. et al. Reversal of spontaneous autoimmune insulitis in nonobese diabetic mice by soluble lymphotoxin receptor. J. Exp. Med. 193, 1327–1332 (2001)
Acknowledgements
We thank C. Sigurdson and M. Zabel for critical reading of the manuscript, and R. Zinkernagel for support. This work was supported by grants of the Bundesamt für Bildung und Wissenschaft, the Swiss National Foundation, the NCCR on neural plasticity and repair, and the Migros foundation to A.A. M.P. was a postdoctoral fellow of the Deutsche Forschungsgemeinschaft. M.H. is supported by a generous educational grant of the Catello family and by the Verein zur Förderung des Akademischen Nachwuchses. F.L.H. is supported by the Stammbach Foundation and by the Bonizzi-Theler Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Prinz, M., Heikenwalder, M., Junt, T. et al. Positioning of follicular dendritic cells within the spleen controls prion neuroinvasion. Nature 425, 957–962 (2003). https://doi.org/10.1038/nature02072
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature02072
This article is cited by
-
Unaltered intravenous prion disease pathogenesis in the temporary absence of marginal zone B cells
Scientific Reports (2019)
-
Prions, prionoids and protein misfolding disorders
Nature Reviews Genetics (2018)
-
Cellular mechanisms responsible for cell-to-cell spreading of prions
Cellular and Molecular Life Sciences (2018)
-
Enhanced neuroinvasion by smaller, soluble prions
Acta Neuropathologica Communications (2017)
-
Follicular dendritic cells: dynamic antigen libraries
Nature Reviews Immunology (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.