Abstract
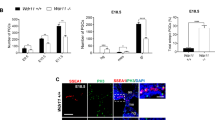
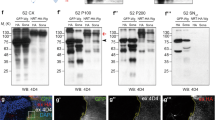
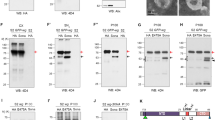
Intraflagellar transport (IFT) proteins were first identified as essential factors for the growth and maintenance of flagella in the single-celled alga Chlamydomonas reinhardtii1. In a screen for embryonic patterning mutations induced by ethylnitrosourea, here we identify two mouse mutants, wimple (wim) and flexo (fxo), that lack ventral neural cell types and show other phenotypes characteristic of defects in Sonic hedgehog signalling. Both mutations disrupt IFT proteins: the wim mutation is an allele of the previously uncharacterized mouse homologue of IFT172; and fxo is a new hypomorphic allele of polaris, the mouse homologue of IFT88. Genetic analysis shows that Wim, Polaris and the IFT motor protein Kif3a are required for Hedgehog signalling at a step downstream of Patched1 (the Hedgehog receptor) and upstream of direct targets of Hedgehog signalling. Our data show that IFT machinery has an essential and vertebrate-specific role in Hedgehog signal transduction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rosenbaum, J. L. & Witman, G. B. Intraflagellar transport. Nature Rev. Mol. Cell Biol. 3, 813–825 (2002)
Ma, Y. et al. Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of Dispatched. Cell 111, 63–75 (2002)
Caspary, T. et al. Mouse Dispatched homolog1 is required for long-range, but not juxtacrine, Hh signaling. Curr. Biol. 12, 1628–1632 (2002)
Murcia, N. S. et al. The Oak Ridge polycystic kidney (orpk) disease gene is required for left–right axis determination. Development 127, 2347–2355 (2000)
Collignon, J., Varlet, I. & Robertson, E. J. Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature 381, 155–158 (1996)
Schimenti, J. C. et al. Interdigitated deletion complexes on mouse chromosome 5 induced by irradiation of embryonic stem cells. Genome Res. 10, 1043–1050 (2000)
Motoyama, J. et al. Differential requirement for Gli2 and Gli3 in ventral neural cell fate specification. Dev. Biol. 259, 150–161 (2003)
Chiang, C. et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407–413 (1996)
Litingtung, Y. & Chiang, C. Control of Shh activity and signaling in the neural tube. Dev. Dyn. 219, 143–154 (2000)
Takeda, S. et al. Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A-/- mice analysis. J. Cell Biol. 145, 825–836 (1999)
Marszalek, J. R., Ruiz-Lozano, P., Roberts, E., Chien, K. R. & Goldstein, L. S. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc. Natl Acad. Sci. USA 96, 5043–5048 (1999)
Goodrich, L. V., Milenkovic, L., Higgins, K. M. & Scott, M. P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277, 1109–1113 (1997)
Eggenschwiler, J. T., Espinoza, E. & Anderson, K. V. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature 412, 194–198 (2001)
Eggenschwiler, J. T. & Anderson, K. V. Dorsal and lateral fates in the mouse neural tube require the cell-autonomous activity of the open brain gene. Dev. Biol. 227, 648–660 (2000)
Wang, B., Fallon, J. F. & Beachy, P. A. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100, 423–434 (2000)
Litingtung, Y. & Chiang, C. Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3. Nature Neurosci. 3, 979–985 (2000)
Wheatley, D. N., Wang, A. M. & Strugnell, G. E. Expression of primary cilia in mammalian cells. Cell Biol. Int. 20, 73–81 (1996)
Sisson, J. C., Ho, K. S., Suyama, K. & Scott, M. P. Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell 90, 235–245 (1997)
Ray, K. et al. Kinesin-II is required for axonal transport of choline acetyltransferase in Drosophila. J. Cell Biol. 147, 507–518 (1999)
Han, Y., Kwok, B. H. & Kernan, M. J. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr. Biol. 13, 1679–1686 (2003)
Bale, A. E. Hedgehog signaling and human disease. Annu. Rev. Genom. Hum. Genet. 3, 47–65 (2002)
Sloboda, R. D. A healthy understanding of intraflagellar transport. Cell Motil. Cytoskeleton 52, 1–8 (2002)
Kasarskis, A., Manova, K. & Anderson, K. V. A phenotype-based screen for embryonic lethal mutations in the mouse. Proc. Natl Acad. Sci. USA 95, 7485–7490 (1998)
Hui, C. C. & Joyner, A. L. A mouse model of Greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nature Genet. 3, 241–246 (1993)
Cole, T. B., Wenzel, H. J., Kafer, K. E., Schwartzkroin, P. A. & Palmiter, R. D. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl Acad. Sci. USA 96, 1716–1721 (1999)
Vetter, D. E. et al. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nature Genet. 31, 363–369 (2002)
Weiher, H., Noda, T., Gray, D. A., Sharpe, A. H. & Jaenisch, R. Transgenic mouse model of kidney disease: insertional inactivation of ubiquitously expressed gene leads to nephrotic syndrome. Cell 62, 425–434 (1990)
Farrelly, D. et al. Mice mutant for glucokinase regulatory protein exhibit decreased liver glucokinase: a sequestration mechanism in metabolic regulation. Proc. Natl Acad. Sci. USA 96, 14511–14516 (1999)
Howard, P. W. & Maurer, R. A. Identification of a conserved protein that interacts with specific LIM homeodomain transcription factors. J. Biol. Chem. 275, 13336–13342 (2000)
Sulik, K. et al. Morphogenesis of the murine node and notochordal plate. Dev. Dyn. 201, 260–278 (1994)
Acknowledgements
We thank K. Maxwell and T. Caspary for initial experiments with fxo; N. Lampen for assistance with SEM; J. Eggenschwiler, D. Cole and M. Kernan for sharing unpublished data; C. Sander and B. Reva for discussions about Wim and Polaris protein structures; T. Bestor, T. Caspary, J. Eggenschwiler, M. García-García and J. Lee for comments on the manuscript; E. Robertson, M. Scott and J. Schimenti for mice; and C. Cepko for the Chx10 antibody. Monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank, which was developed under the auspices of the National Institute of Child Health and Human Development and is maintained by The University of Iowa, Department of Biological Sciences. Genome sequence analysis used Ensembl and the Celera Discovery System and associated databases, made possible in part by the AMDeC Foundation. This work was supported by NIH grants to K.V.A. and the Lita Annenberg Hazen Foundation. L.N. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Huangfu, D., Liu, A., Rakeman, A. et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83–87 (2003). https://doi.org/10.1038/nature02061
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02061
This article is cited by
-
Hedgehog signalling is involved in acquired resistance to KRASG12C inhibitors in lung cancer cells
Cell Death & Disease (2024)
-
Absence of the primary cilia formation gene Talpid3 impairs muscle stem cell function
Communications Biology (2023)
-
Primary cilia control cellular patterning of Meibomian glands during morphogenesis but not lipid composition
Communications Biology (2023)
-
Tau tubulin kinase 1 and 2 regulate ciliogenesis and human pluripotent stem cells–derived neural rosettes
Scientific Reports (2023)
-
Coordination of canonical and noncanonical Hedgehog signalling pathways mediated by WDR11 during primordial germ cell development
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.