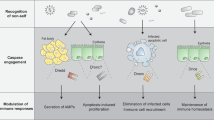
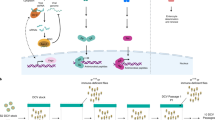
Abstract
Drosophila mounts a potent host defence when challenged by various microorganisms. Analysis of this defence by molecular genetics has now provided a global picture of the mechanisms by which this insect senses infection, discriminates between various classes of microorganisms and induces the production of effector molecules, among which antimicrobial peptides are prominent. An unexpected result of these studies was the discovery that most of the genes involved in the Drosophila host defence are homologous or very similar to genes implicated in mammalian innate immune defences. Recent progress in research on Drosophila immune defence provides evidence for similarities and differences between Drosophila immune responses and mammalian innate immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Medzhitov, R. & Janeway, C. Jr Innate immunity. N. Engl. J. Med. 343, 338–344 (2000)
Janeway, C. A. Jr & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002)
Rizki, R. M. & Rizki, T. M. Selective destruction of a host blood cell type by a parasitoid wasp. Proc. Natl Acad. Sci. USA 81, 6154–6158 (1984)
Braun, A., Hoffmann, J. A. & Meister, M. Analysis of the Drosophila host defense in domino mutant larvae, which are devoid of hemocytes. Proc. Natl Acad. Sci. USA 95, 14337–14342 (1998)
Hoffmann, J. A. & Reichhart, J. M. Drosophila innate immunity: an evolutionary perspective. Nature Immunol. 3, 121–126 (2002)
Ferrandon, D. et al. A drosomycin–GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J. 17, 1217–1227 (1998)
Tzou, P. et al. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity 13, 737–748 (2000)
Bulet, P., Hetru, C., Dimarcq, J. L. & Hoffmann, D. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 23, 329–344 (1999)
Engstrom, Y. et al. κB-like motifs regulate the induction of immune genes in Drosophila. J. Mol. Biol. 232, 327–333 (1993)
Kappler, C. et al. Insect immunity. Two 17 bp repeats nesting a κB-related sequence confer inducibility to the diptericin gene and bind a polypeptide in bacteria-challenged Drosophila. EMBO J. 12, 1561–1568 (1993)
Nusslein-Volhard, C., Lohs-Schardin, M., Sander, K. & Cremer, C. A dorso-ventral shift of embryonic primordia in a new maternal-effect mutant of Drosophila. Nature 283, 474–476 (1980)
St Johnston, D. & Nüsslein-Volhard, C. The origin of pattern and polarity in the Drosophila embryo. Cell 68, 201–219 (1992)
Morisato, D. & Anderson, K. V. Signaling pathways that establish the dorsal–ventral pattern of the Drosophila embryo. Annu. Rev. Genet. 29, 371–399 (1995)
Belvin, M. P. & Anderson, K. V. A conserved signaling pathway: the Drosophila Toll–Dorsal pathway. Annu. Rev. Cell Dev. Biol. 12, 393–416 (1996)
Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J. M. & Hoffmann, J. A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983 (1996)
Tobias, P. S., Soldau, K. & Ulevitch, R. J. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J. Biol. Chem. 264, 10867–10871 (1989)
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C. A. Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997)
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998)
Qureshi, S. T. et al. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189, 615–625 (1999)
Rock, F. L., Hardiman, G., Timans, J. C., Kastelein, R. A. & Bazan, J. F. A family of human receptors structurally related to Drosophila Toll. Proc. Natl Acad. Sci. USA 95, 588–593 (1998)
Rutschmann, S., Kilinc, A. & Ferrandon, D. Cutting edge: the Toll pathway is required for resistance to Gram-positive bacterial infections in Drosophila. J. Immunol. 168, 1542–1546 (2002)
Lemaitre, B. et al. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc. Natl Acad. Sci. USA 92, 9465–9469 (1995)
Mizuguchi, K., Parker, J. S., Blundell, T. L. & Gay, N. J. Getting knotted: a model for the structure and activation of Spatzle. Trends Biochem. Sci. 23, 239–242 (1998)
DeLotto, Y. & DeLotto, R. Proteolytic processing of the Drosophila Spatzle protein by easter generates a dimeric NGF-like molecule with ventralising activity. Mech. Dev. 72, 141–148 (1998)
Weber, A. N. R. et al. Binding of the Drosophila cytokine Spaetzle to Toll is direct and establishes signaling. Nature Immunol. 8, 794–800 (2003)
Horng, T. & Medzhitov, R. Drosophila MyD88 is an adapter in the Toll signaling pathway. Proc. Natl Acad. Sci. USA 98, 12654–12658 (2001)
Sun, H., Bristow, B. N., Qu, G. & Wasserman, S. A. A heterotrimeric death domain complex in Toll signaling. Proc. Natl Acad. Sci. USA 99, 12871–12876 (2002)
Tauszig-Delamasure, S., Bilak, H., Capovilla, M., Hoffmann, J. A. & Imler, J. L. Drosophila MyD88 is required for the response to fungal and Gram-positive bacterial infections. Nature Immunol. 3, 91–97 (2002)
Charatsi, I., Luschnig, S., Bartoszewski, S., Nusslein-Volhard, C. & Moussian, B. Krapfen/dMyd88 is required for the establishment of dorsoventral pattern in the Drosophila embryo. Mech. Dev. 120, 219–226 (2003)
Janssens, S. & Beyaert, R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol. Cell 11, 293–302 (2003)
Ip, Y. T. et al. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell 75, 753–763 (1993)
Meng, X., Khanuja, B. S. & Ip, Y. T. Toll receptor-mediated Drosophila immune response requires Dif, an NF-κB factor. Genes Dev. 13, 792–797 (1999)
Manfruelli, P., Reichhart, J. M., Steward, R., Hoffmann, J. A. & Lemaitre, B. A mosaic analysis in Drosophila fat body cells of the control of antimicrobial peptide genes by the Rel proteins Dorsal and DIF. EMBO J. 18, 3380–3391 (1999)
Rutschmann, S. et al. The Rel protein DIF mediates the antifungal but not the antibacterial host defense in Drosophila. Immunity 12, 569–580 (2000)
Nicolas, E., Reichhart, J. M., Hoffmann, J. A. & Lemaitre, B. In vivo regulation of the IκB homologue cactus during the immune response of Drosophila. J. Biol. Chem. 273, 10463–10469 (1998)
Fernandez, N. Q., Grosshans, J., Goltz, J. S. & Stein, D. Separable and redundant regulatory determinants in Cactus mediate its dorsal group dependent degradation. Development 128, 2963–2974 (2001)
Drier, E. A., Huang, L. H. & Steward, R. Nuclear import of the Drosophila Rel protein Dorsal is regulated by phosphorylation. Genes Dev. 13, 556–568 (1999)
Avila, A., Silverman, N., Diaz-Meco, M. T. & Moscat, J. The Drosophila atypical protein kinase C–ref(2)p complex constitutes a conserved module for signaling in the toll pathway. Mol. Cell. Biol. 22, 8787–8795 (2002)
De Gregorio, E., Spellman, P. T., Rubin, G. M. & Lemaitre, B. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc. Natl Acad. Sci. USA 98, 12590–12595 (2001)
Irving, P. et al. A genome-wide analysis of immune responses in Drosophila. Proc. Natl Acad. Sci. USA 98, 15119–15124 (2001)
Tauszig, S., Jouanguy, E., Hoffmann, J. A. & Imler, J. L. Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc. Natl Acad. Sci. USA 97, 10520–10525 (2000)
Kambris, Z., Hoffmann, J. A., Imler, J. L. & Capovilla, M. Tissue and stage-specific expression of the Tolls in Drosophila embryos. Gene Expr. Patterns 2, 311–317 (2002)
Parker, J. S., Mizuguchi, K. & Gay, N. J. A family of proteins related to Spatzle, the toll receptor ligand, are encoded in the Drosophila genome. Proteins 45, 71–80 (2001)
Keith, F. J. & Gay, N. J. The Drosophila membrane receptor Toll can function to promote cellular adhesion. EMBO J. 9, 4299–4306 (1990)
Georgel, P. et al. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev. Cell 1, 503–514 (2001)
Dushay, M. S., Asling, B. & Hultmark, D. Origins of immunity: Relish, a compound Rel-like gene in the antibacterial defense of Drosophila. Proc. Natl Acad. Sci. USA 93, 10343–10347 (1996)
Hedengren, M. et al. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol. Cell 4, 827–837 (1999)
Stoven, S., Ando, I., Kadalayil, L., Engstrom, Y. & Hultmark, D. Activation of the Drosophila NF-κB factor Relish by rapid endoproteolytic cleavage. EMBO Rep. 1, 347–352 (2000)
Naitza, S. et al. The Drosophila immune defense against Gram-negative infection requires the death protein dFADD. Immunity 17, 575–581 (2002)
Leulier, F., Vidal, S., Saigo, K., Ueda, R. & Lemaitre, B. Inducible expression of double-stranded RNA reveals a role for dFADD in the regulation of the antibacterial response in Drosophila adults. Curr. Biol. 12, 996–1000 (2002)
Vidal, S. et al. Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-κB-dependent innate immune responses. Genes Dev. 15, 1900–1912 (2001)
Rutschmann, S. et al. Role of Drosophila IKKγ in a Toll-independent antibacterial immune response. Nature Immunol. 1, 342–347 (2000)
Lu, Y., Wu, L. P. & Anderson, K. V. The antibacterial arm of the Drosophila innate immune response requires an IκB kinase. Genes Dev. 15, 104–110 (2001)
Silverman, N. et al. A Drosophila IκB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev. 14, 2461–2471 (2000)
Leulier, F., Rodriguez, A., Khush, R. S., Abrams, J. M. & Lemaitre, B. The Drosophila caspase Dredd is required to resist Gram-negative bacterial infection. EMBO Rep. 1, 353–358 (2000)
Elrod-Erickson, M., Mishra, S. & Schneider, D. Interactions between the cellular and humoral immune responses in Drosophila. Curr. Biol. 10, 781–784 (2000)
Hu, S. & Yang, X. dFADD, a novel death domain-containing adapter protein for the Drosophila caspase DREDD. J. Biol. Chem. 275, 30761–30764 (2000)
Stoven, S. et al. Caspase-mediated processing of the Drosophila NF-κB factor Relish. Proc. Natl Acad. Sci. USA 100, 5991–5996 (2003)
Boutros, M., Agaisse, H. & Perrimon, N. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev. Cell 3, 711–722 (2002)
De Gregorio, E., Spellman, P. T., Tzou, P., Rubin, G. M. & Lemaitre, B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 21, 2568–2579 (2002)
Michel, T., Reichhart, J. M., Hoffmann, J. A. & Royet, J. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 414, 756–759 (2001)
Gobert, V. et al. Toll activation during bacterial infection in Drosophila requires the concomitant function of two distinct blood-borne recognition proteins. Science (submitted)
Yoshida, H., Kinoshita, K. & Ashida, M. Purification of a peptidoglycan recognition protein from hemolymph of the silkworm, Bombyx mori. J. Biol. Chem. 271, 13854–13860 (1996)
Lee, W. J., Lee, J. D., Kravchenko, V. V., Ulevitch, R. J. & Brey, P. T. Purification and molecular cloning of an inducible Gram-negative bacteria-binding protein from the silkworm, Bombyx mori. Proc. Natl Acad. Sci. USA 93, 7888–7893 (1996)
Kang, D., Liu, G., Lundstrom, A., Gelius, E. & Steiner, H. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc. Natl Acad. Sci. USA 95, 10078–10082 (1998)
Ochiai, M. & Ashida, M. A pattern recognition protein for peptidoglycan. Cloning the cDNA and the gene of the silkworm, Bombyx mori. J. Biol. Chem. 274, 11854–11858 (1999)
Werner, T. et al. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc. Natl Acad. Sci. USA 97, 13772–13777 (2000)
Ochiai, M. & Ashida, M. A pattern-recognition protein for β-1,3-glucan. The binding domain and the cDNA cloning of β-1,3-glucan recognition protein from the silkworm, Bombyx mori. J. Biol. Chem. 275, 4995–5002 (2000)
Ligoxygakis, P., Pelte, N., Hoffmann, J. A. & Reichhart, J. M. Activation of Drosophila Toll during fungal infection by a blood serine protease. Science 297, 114–116 (2002)
Gottar, M. et al. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 416, 640–644 (2002)
Choe, K. M., Werner, T., Stoven, S., Hultmark, D. & Anderson, K. V. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science 296, 359–362 (2002)
Ramet, M., Manfruelli, P., Pearson, A., Mathey-Prevot, B. & Ezekowitz, R. A. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 416, 644–648 (2002)
Takehana, A. et al. Overexpression of a pattern-recognition receptor, peptidoglycan-recognition protein-LE, activates imd/relish-mediated antibacterial defense and the prophenoloxidase cascade in Drosophila larvae. Proc. Natl Acad. Sci. USA 99, 13705–13710 (2002)
Cheng, X., Zhang, X., Pflugrath, J. W. & Studier, F. W. The structure of bacteriophage T7 lysozyme, a zinc amidase and an inhibitor of T7 RNA polymerase. Proc. Natl Acad. Sci. USA 91, 4034–4038 (1994)
Liepinsh, E., Genereux, C., Dehareng, D., Joris, B. & Otting, G. NMR structure of Citrobacter freundii AmpD, comparison with bacteriophage T7 lysozyme and homology with PGRP domains. J. Mol. Biol. 327, 833–842 (2003)
Mellroth, P., Karlsson, J. & Steiner, H. A scavenger function for a Drosophila peptidoglycan recognition protein. J. Biol. Chem. 278, 7059–7064 (2003)
Kim, M. S., Byun, M. & Oh, B. H. Crystal structure of peptidoglycan recognition protein LB from Drosophila melanogaster. Nature Immunol. 4, 787–793 (2003)
Leulier, F. et al. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nature Immunol. 4, 478–484 (2003)
Liu, C., Xu, Z., Gupta, D. & Dziarski, R. Peptidoglycan recognition proteins: a novel family of four human innate immunity pattern recognition molecules. J. Biol. Chem. 276, 34686–34694 (2001)
Dziarski, R., Platt, K. A., Gelius, E., Steiner, H. & Gupta, D. Defect in neutrophil killing and increased susceptibility to infection with non-pathogenic Gram-positive bacteria in peptidoglycan recognition protein-S (PGRP-S)-deficient mice. Blood 102, 689–697 (2003)
Takeuchi, O. et al. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 11, 443–451 (1999)
Gutierrez, O. et al. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-κB activation. J. Biol. Chem. 277, 41701–41705 (2002)
Girardin, S. E. et al. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science 300, 1584–1587 (2003)
Becker, M. N., Diamond, G., Verghese, M. W. & Randell, S. H. CD14-dependent lipopolysaccharide-induced β-defensin-2 expression in human tracheobronchial epithelium. J. Biol. Chem. 275, 29731–29736 (2000)
Liu, L., Roberts, A. A. & Ganz, T. By IL-1 signaling, monocyte-derived cells dramatically enhance the epidermal antimicrobial response to lipopolysaccharide. J. Immunol. 170, 575–580 (2003)
Acknowledgements
The author acknowledges invaluable exchanges with C. A. Janeway, R. A. Ezekowitz, F. Kafatos, T. Ganz and B. Beutler over many years, as well as the strongly motivating influence of H. G. Boman in the early period of these studies. I am particularly indebted to present and former associates of this group, namely P. Bulet, J. L. Dimarcq, D. Ferrandon, C. Hetru, D. Hoffmann, J. L. Imler, M. Lagueux, B. Lemaitre, E. Levashina, M. Meister, J. M. Reichhart and J. Royet.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares that he has no competing financial interests.
Rights and permissions
About this article
Cite this article
Hoffmann, J. The immune response of Drosophila. Nature 426, 33–38 (2003). https://doi.org/10.1038/nature02021
Issue Date:
DOI: https://doi.org/10.1038/nature02021
This article is cited by
-
Drosophila eIF3f1 mediates host immune defense by targeting dTak1
EMBO Reports (2024)
-
Hemocytes of a tropical midge Chironomus ramosus (Diptera: Chironomidae)
International Journal of Tropical Insect Science (2024)
-
Toll-like receptor 4 (TLR4): new insight immune and aging
Immunity & Ageing (2023)
-
Effects of TmTak1 silencing on AMP production as an Imd pathway component in Tenebrio molitor
Scientific Reports (2023)
-
Structural and functional studies of pattern recognition receptors βGRP1 and βGRP2 in Sogatella furcifera
Applied Entomology and Zoology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.