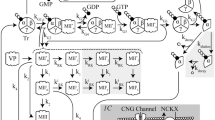
Abstract
Retinal rods and cones share a phototransduction pathway involving cyclic GMP1. Cones are typically 100 times less photosensitive than rods and their response kinetics are several times faster2, but the underlying mechanisms remain largely unknown. Almost all proteins involved in phototransduction have distinct rod and cone variants. Differences in properties between rod and cone pigments have been described, such as a 10-fold shorter lifetime of the meta-II state (active conformation) of cone pigment3,4,5,6 and its higher rate of spontaneous isomerization7,8, but their contributions to the functional differences between rods and cones remain speculative. We have addressed this question by expressing human or salamander red cone pigment in Xenopus rods, and human rod pigment in Xenopus cones. Here we show that rod and cone pigments when present in the same cell produce light responses with identical amplification and kinetics, thereby ruling out any difference in their signalling properties. However, red cone pigment isomerizes spontaneously 10,000 times more frequently than rod pigment. This high spontaneous activity adapts the native cones even in darkness, making them less sensitive and kinetically faster than rods. Nevertheless, additional factors are probably involved in these differences.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Yau, K. W. Phototransduction mechanism in retinal rods and cones. The Friedenwald Lecture. Invest. Ophthalmol. Vis. Sci. 35, 9–32 (1994)
Baylor, D. A. Photoreceptor signals and vision. The Proctor Lecture. Invest. Ophthalmol. Vis. Sci. 28, 34–49 (1987)
Okada, T. et al. Circular dichroism of metaiodopsin II and its binding to transducin: a comparative study between meta II intermediates of iodopsin and rhodopsin. Biochemistry 33, 4940–4946 (1994)
Imai, H., Imamoto, Y., Yoshizawa, T. & Shichida, Y. Difference in molecular properties between chicken green and rhodopsin as related to the functional difference between cone and rod photoreceptor cells. Biochemistry 34, 10525–10531 (1995)
Imai, H., Terakita, A., Tachibanaki, S., Imamoto, Y., Yoshizawa, T. & Shichida, Y. Photochemical and biochemical properties of chicken blue-sensitive cone visual pigment. Biochemistry 36, 12773–12779 (1997)
Starace, D. M. & Knox, B. E. Activation of transducin by a Xenopus short wavelength visual pigment. J. Biol. Chem. 272, 1095–1100 (1997)
Barlow, H. B. in Visual Problems of Colour Vol. 2, 617–630 (HM Stationery Office, London, 1958)
Rieke, F. & Baylor, D. A. Origin and functional impact of dark noise in retinal cones. Neuron 26, 181–186 (2000)
Witkovsky, P., Levine, J. S., Engbretson, G. A., Hassin, G. & MacNichol, E. F. Jr A microspectrophotometric study of normal and artificial visual pigments in the photoreceptors of Xenopus laevis. Vision Res. 21, 867–873 (1981)
Palacios, A. G., Srivastava, R. & Goldsmith, T. H. Spectral and polarization sensitivity of photocurrents of amphibian rods in the visible and ultraviolet. Vis. Neurosci. 15, 319–331 (1998)
Govardovskii, V. I., Fyhrquist, N., Reuter, T., Kuzmin, D. G. & Donner, K. In search of the visual pigment template. Vis. Neurosci. 17, 509–528 (2000)
Moritz, O. L., Tam, B. M., Papermaster, D. S. & Nakayama, T. A functional rhodopsin–green fluorescent protein fusion protein localizes correctly in transgenic Xenopus laevis retinal rods and is expressed in a time-dependent pattern. J. Biol. Chem. 276, 28242–28251 (2001)
Lyubarsky, A. L., Chen, C.-K., Simon, M. I. & Pugh, E. N. Jr Mice lacking G-protein receptor kinase 1 have profoundly slowed recovery of cone-driven retinal responses. J. Neurosci. 20, 2209–2217 (2000)
Baylor, D. A., Matthews, G. & Yau, K.-W. Two components of electrical dark noise in toad retinal rod outer segments. J. Physiol. (Lond.) 309, 591–621 (1980)
Rieke, F. & Baylor, D. A. Molecular origin of continuous dark noise in rod photoreceptors. Biophys. J. 71, 2553–2572 (1996)
Katz, B. & Miledi, R. The statistical nature of the acetylcholine potential and its molecular components. J. Physiol. (Lond.) 224, 665–699 (1972)
Sampath, A. P. & Baylor, D. A. Molecular mechanism of spontaneous pigment activation in retinal cones. Biophys. J. 83, 184–193 (2002)
Nakatani, K. & Yau, K.-W. Sodium-dependent calcium extrusion and sensitivity regulation in retinal cones of the salamander. J. Physiol. (Lond.) 409, 525–548 (1989)
Ma, J.-X. et al. A visual pigment expressed in both rod and cone photoreceptors. Neuron 32, 451–461 (2001)
Miller, J. L. & Korenbrot, J. I. Phototransduction and adaptation in rods, single cones, and twin cones of the striped bass retina: a comparative study. Vis. Neurosci. 10, 653–667 (1993)
Tachibanaki, S., Tsushima, S. & Kawamura, S. Low amplification and fast visual pigment phosphorylation as mechanisms characterizing cone photoresponses. Proc. Natl Acad. Sci. USA 98, 14044–14049 (2001)
Miller, J. L., Picones, A. & Korenbrot, J. I. Differences in transduction between rod and cone photoreceptors: an exploration of the role of calcium homeostasis. Curr. Opin. Neurobiol. 4, 488–495 (1994)
Kroll, K. L. & Amaya, E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signalling requirements during gastrulation. Development 122, 3173–3183 (1996)
Wang, Y. et al. A locus control region adjacent to the human red and green visual pigment genes. Neuron 9, 429–440 (1992)
Makino, C. L., Groesbeek, M., Lugtenburg, J. & Baylor, D. A. Spectral tuning in salamander visual pigments studied with dihydroretinal chromophores. Biophys. J. 77, 1024–1035 (1999)
Merbs, S. L. & Nathans, J. Absorption spectra of human cone pigments. Nature 356, 433–435 (1992)
Whitmore, A. V. & Bowmaker, J. K. Seasonal variation in cone sensitivity and short-wave absorbing visual pigments in the rudd Scardinius erythrophthalmus. J. Comp. Physiol. A 166, 103–115 (1989)
Bowmaker, J. K. & Dartnall, H. J. Visual pigments of rods and cones in a human retina. J. Physiol. (Lond.) 298, 501–511 (1980)
Harosi, F. I. An analysis of two spectral properties of vertebrate visual pigments. Vision Res. 34, 1359–1367 (1994)
Hárosi, F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J. Gen. Physiol. 66, 357–382 (1975)
Acknowledgements
We thank J. Lai, Y. Liang and R. Bruno for help with generating transgenic frogs; J. Nathans for the human rhodopsin and red cone opsin cDNAs and the antibody to red opsin; V. Bhandawat for suggesting the program for computer simulations of random events; and D. A. Baylor, M. E. Burns, S. Kawamura, E. N. Pugh Jr, F. Rieke, J. L. Schnapf, Y. Shichida and members of the Yau laboratory for discussions and comments on the manuscript. This work was supported by the US National Eye Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Kefalov, V., Fu, Y., Marsh-Armstrong, N. et al. Role of visual pigment properties in rod and cone phototransduction. Nature 425, 526–531 (2003). https://doi.org/10.1038/nature01992
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01992
This article is cited by
-
Investigating the Ca2+-dependent and Ca2+-independent mechanisms for mammalian cone light adaptation
Scientific Reports (2018)
-
Adaptation of pineal expressed teleost exo-rod opsin to non-image forming photoreception through enhanced Meta II decay
Cellular and Molecular Life Sciences (2011)
-
Quantal noise from human red cone pigment
Nature Neuroscience (2008)
-
Phototransduction in mouse rods and cones
Pflügers Archiv - European Journal of Physiology (2007)
-
Gene silencing in Xenopus laevis by DNA vector-based RNA interference and transgenesis
Cell Research (2006)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.