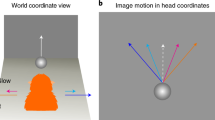
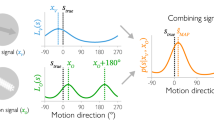
Abstract
Current views of the visual system assume that the primate brain analyses form and motion along largely independent pathways1; they provide no insight into why form is sometimes interpreted as motion. In a series of psychophysical and electrophysiological experiments in humans and macaques, here we show that some form information is processed in the prototypical motion areas of the superior temporal sulcus (STS). First, we show that STS cells respond to dynamic Glass patterns2, which contain no coherent motion but suggest a path of motion3. Second, we show that when motion signals conflict with form signals suggesting a different path of motion, both humans and monkeys perceive motion in a compromised direction. This compromise also has a correlate in the responses of STS cells, which alter their direction preferences in the presence of conflicting implied motion information. We conclude that cells in the prototypical motion areas in the dorsal visual cortex process form that implies motion. Estimating motion by combining motion cues with form cues may be a strategy to deal with the complexities of motion perception in our natural environment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mishkin, M., Ungerleider, L. & Macko, K. Object vision and spatial vision: two central pathways. Trends Neurosci. 6, 414–417 (1983)
Glass, L. Moiré effect from random dots. Nature 223, 578–580 (1969)
Ross, J., Badcock, D. R. & Hayes, A. Coherent global motion in the absence of coherent velocity signals. Curr. Biol. 10, 679–682 (2000)
Geisler, W. S. Motion streaks provide a spatial code for motion direction. Nature 400, 65–69 (1999)
Geisler, W. S., Albrecht, D. G., Crane, A. M. & Stern, L. Motion direction signals in the primary visual cortex of cat and monkey. Vis. Neurosci. 18, 501–516 (2001)
Jancke, D. Orientation formed by a spot's trajectory: A two-dimensional population approach in primary visual cortex. J. Neurosci. 20, RC86 (1–6) (2000)
Kourtzi, Z. & Kanwisher, N. Activation in human MT/MST by static images with implied motion. J. Cogn. Neurosci. 12, 48–55 (2000)
Wilson, H. R., Wilkinson, F. & Asaad, W. Concentric orientation summation in human form vision. Vision Res. 37, 2325–2330 (1997)
Burr, D. C. & Ross, J. Direct evidence that ‘Speedlines’ influence motion mechanisms. J. Neurosci. 22, 8661–8664 (2002)
Smith, M. A., Bair, W. & Movshon, J. A. Signals in macaque striate cortical neurons that support the perception of Glass patterns. J. Neurosci. 22, 8334–8345 (2002)
Schoppmann, A. & Hoffmann, K.-P. Continuous mapping of direction selectivity in the cat's visual cortex. Neurosci. Lett. 2, 177–181 (1976)
Bremmer, F., Ilg, U. J., Thiele, A., Distler, C. & Hoffmann, K. P. Eye position effects in monkey cortex. I. Visual and pursuit-related activity in extrastriate areas MT and MST. J. Neurophysiol. 77, 944–961 (1997)
Snowden, R. J., Treue, S., Erickson, R. G. & Andersen, R. A. The response of area MT and V1 neurons to transparent motion. J. Neurosci. 11, 2768–2785 (1991)
Qian, N. & Andersen, R. A. Transparent motion perception as detection of unbalanced motion signals. II. Physiology. J. Neurosci. 14, 7367–7380 (1994)
Maunsell, J. H. & Van Essen, D. C. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J. Neurophysiol. 49, 1127–1147 (1983)
Albright, T. D. Direction and orientation selectivity of neurons in visual area MT of the macaque. J. Neurophysiol. 52, 1106–1130 (1984)
Pack, C. & Born, R. T. Temporal dynamics of a neural solution to the aperture problem in visual area MT of macaque brain. Nature 409, 1040–1042 (2001)
Newsome, W. T. & Pare, E. B. A selective impairment of motion perception following lesions of the middle temporal visual area (MT). J. Neurosci. 8, 2201–2211 (1988)
Rudolph, K. & Pasternak, T. Transient and permanent deficits in motion perception after lesions of cortical areas MT and MST in the macaque monkey. Cereb. Cortex 9, 90–100 (1999)
Batschelet, E. Circular Statistics in Biology (Academic, London, 1981)
Acknowledgements
We thank J. Constanza, D. Diep, L. Abavare and M. Bronzel for technical assistance, C. Distler for the surgeries, and G. Stoner and T. Albright for comments and discussions. The Human Frontiers Science Program and the Australian Research Council supported this work financially.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Krekelberg, B., Dannenberg, S., Hoffmann, KP. et al. Neural correlates of implied motion. Nature 424, 674–677 (2003). https://doi.org/10.1038/nature01852
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01852
This article is cited by
-
Attention influences the effects of the previous form orientation on the current motion direction estimation
Scientific Reports (2024)
-
Heading representations in primates are compressed by saccades
Nature Communications (2017)
-
Implied motion perception from a still image in infancy
Experimental Brain Research (2014)
-
Ventral and dorsal streams processing visual motion perception (FDG-PET study)
BMC Neuroscience (2012)
-
Long- and short-term plastic modeling of action prediction abilities in volleyball
Psychological Research (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.