Abstract
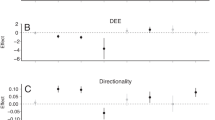
A central issue in ecology lies in identifying the importance of resources, natural enemies and behaviour in the regulation of animal populations. Much of the debate on this subject has focused on animals that show cyclic fluctuations in abundance1,2,3,4,5,6,7. However, there is still disagreement about the role of extrinsic (food, parasites or predators) and intrinsic (behaviour) factors in causing cycles2,8,9,10. Recent studies have examined the impact of natural enemies1,3,4,7, although spatial patterns resulting from restricted dispersal or recruitment are increasingly recognized as having the potential to influence unstable population dynamics5,6,11,12,13. We tested the hypothesis that population cycles in a territorial bird, red grouse Lagopus lagopus scoticus, are caused by delayed density-dependent changes in the aggressiveness and spacing behaviour of males. Here we show that increasing aggressiveness experimentally for a short period in autumn reduced recruitment and subsequent breeding density by 50%, and changed population trajectories from increasing to declining. Intrinsic processes can therefore have fundamental effects on population dynamics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Krebs, C. J. et al. Impact of food and predation on the snowshoe hare cycle. Science 269, 1112–1115 (1995)
Stenseth, N. C., Bjornstad, O. N. & Falck, W. Is spacing behaviour coupled with predation causing the microtine density cycle? A synthesis of current process-oriented and pattern-oriented studies. Proc. R. Soc. Lond. B 263, 1423–1435 (1996)
Hudson, P. J., Dobson, A. P. & Newborn, D. Prevention of population cycles by parasite removal. Science 282, 2256–2258 (1998)
Turchin, P., Taylor, A. D. & Reeve, J. D. Dynamical role of predators in population cycles of a forest insect: An experimental test. Science 285, 1068–1071 (1999)
Grenfell, B. T., Bjornstad, O. N. & Kappey, J. Travelling waves and spatial hierarchies in measles epidemics. Nature 414, 716–723 (2001)
Moss, R. & Watson, A. Population cycles in birds of the grouse family (Tetraonidae). Adv. Ecol. Res. 32, 53–111 (2001)
Korpimaki, E., Norrdahl, K., Klemola, T., Pettersen, T. & Stenseth, N. C. Dynamic effects of predators on cyclic voles: Field experimentation and model extrapolation. Proc. R. Soc. Lond. B 269, 991–997 (2002)
Ostfeld, R. S., Canham, C. D. & Pugh, S. R. Intrinsic density-dependent regulation of vole populations. Nature 366, 259–261 (1993)
Berryman, A. A. in Population Cycles: The Case for Trophic Interactions (ed. Berryman, A. A.) 3–28 (Oxford Univ. Press, Oxford, UK, 2002)
Lambin, X., Krebs, C. J., Moss, R. & Yoccoz, N. G. in Population Cycles: The Case for Trophic Interactions (ed. Berryman, A. A.) 155–176 (Oxford Univ. Press, Oxford, UK, 2002)
Lambin, X. & Yoccoz, N. G. The impact of population kin-structure on nestling survival in Townsend's voles, Microtus townsendii. J. Anim. Ecol. 67, 1–16 (1998)
Bjornstad, O. N. & Grenfell, B. T. Noisy clockwork: Time series analysis of population fluctuations in animals. Science 293, 638–643 (2001)
Blasius, B., Huppert, A. & Stone, L. Complex dynamics and phase synchronization in spatially extended ecological systems. Nature 399, 354–359 (1999)
Mountford, M. C., Watson, A., Moss, R. & Parr, R. in Red Grouse Population Processes (eds Lance, A. N. & Lawton, J. H.) 78–83 (British Ecological Society, Ascot, UK, 1990)
Watson, A., Moss, R., Parr, R., Mountford, M. D. & Rothery, P. Kin landownership, differential aggression between kin and non-kin, and population fluctuations in red grouse. J. Anim. Ecol. 63, 39–50 (1994)
Moss, R., Watson, A. & Parr, R. Experimental prevention of a population cycle in red grouse. Ecology 77, 1512–1530 (1996)
Moss, R., Parr, R. & Lambin, X. Effects of testosterone on breeding density, breeding success and survival of red grouse. Proc. R. Soc. Lond. B 258, 175–180 (1994)
Charnov, E. L. & Finnerty, J. P. Vole population cycles: A case for kin selection. Oecologia 45, 1–2 (1980)
Matthiopoulos, J., Moss, R. & Lambin, X. The kin-facilitation hypothesis for red grouse population cycles: Territory sharing between relatives. Ecol. Model. 127, 53–63 (2000)
Hudson, P. J., Newborn, D. & Robertson, P. J. Seasonal and geographical patterns of mortality in red grouse populations. Wildl. Biol. 2, 79–88 (1997)
Hudson, P. J. et al. Trophic interactions and population growth rates: describing patterns and identifying mechanisms. Phil. Trans. R. Soc. Lond. B 357, 1259–1271 (2002)
Thirgood, S. J., Redpath, S. M., Rothery, P. & Aebischer, N. J. Raptor predation and population limitation in red grouse. J. Anim. Ecol. 69, 504–516 (2000)
Dobson, A. P. & Hudson, P. J. Regulation and stability of a free-living host-parasite system—Trichostrongylus tenuis in red grouse. II. Population models. J. Anim. Ecol. 61, 487–498 (1992)
Hudson, P. J., Newborn, D. & Dobson, A. P. Regulation and stability of a free-living host-parasite system—Trichostrongylus tenuis in red grouse. I. Monitoring and parasite reduction experiments. J. Anim. Ecol. 61, 477–486 (1992)
Hudson, P. J., Dobson, A. P. & Newborn, D. in Ecology and Genetics Of Host–Parasite Interactions (eds Rollisson, D. & Andersson, R. M.) 77–89 (Academic, London, UK, 1985)
Fox, A. & Hudson, P. J. Parasites reduce territorial behaviour in red grouse (Lagopus lagopus scoticus). Ecol. Lett. 4, 139–143 (2001)
Folstad, I. & Karter, A. J. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 139, 603–622 (1992)
Acknowledgements
We thank the British army, English Nature, and the Dalhousie and Dunecht estates for allowing us access to their moors. We particularly thank T. P. J. Helps, C. McCarthy, J. Adamson, D. Calder and A. Dykes for their help in organizing the fieldwork. We also thank R. Cox, N. Green, D. Luccini and J. Irvine for their help with the fieldwork. D. Elston helped with the statistical analyses. S. Albon, M. Harris, X. Lambin, M. Marquiss, R. Moss and R. Van Der Wal made helpful comments on previous drafts of the manuscript. This work was funded by the Natural Environment Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Mougeot, F., Redpath, S., Leckie, F. et al. The effect of aggressiveness on the population dynamics of a territorial bird. Nature 421, 737–739 (2003). https://doi.org/10.1038/nature01395
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01395
This article is cited by
-
Post-whaling shift in mating tactics in male humpback whales
Communications Biology (2023)
-
Is hissing behaviour of incubating great tits related to reproductive investment in the wild?
acta ethologica (2016)
-
Intra-sexual competition modulates calling behavior and its association with secondary sexual traits
Behavioral Ecology and Sociobiology (2016)
-
Eco-evolutionary dynamics: investigating multiple causal pathways linking changes in behavior, population density and natural selection
Journal of Ornithology (2015)
-
A test of the effect of testosterone on a sexually selected carotenoid trait in a cardueline finch
Ecological Research (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.