Abstract
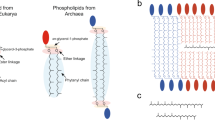
Lipid membranes are essential to the functioning of cells, enabling the existence of concentration gradients of ions and metabolites. Microbial membrane lipids can contain three-, five-, six- and even seven-membered aliphatic rings1,2,3, but four-membered aliphatic cyclobutane rings have never been observed. Here we report the discovery of cyclobutane rings in the dominant membrane lipids of two anaerobic ammonium-oxidizing (anammox) bacteria. These lipids contain up to five linearly fused cyclobutane moieties with cis ring junctions. Such ‘ladderane’ molecules are unprecedented in nature but are known as promising building blocks in optoelectronics4. The ladderane lipids occur in the membrane of the anammoxosome, the dedicated intracytoplasmic compartment where anammox catabolism takes place. They give rise to an exceptionally dense membrane, a tight barrier against diffusion. We propose that such a membrane is required to maintain concentration gradients during the exceptionally slow anammox metabolism and to protect the remainder of the cell from the toxic anammox intermediates. Our results further illustrate that microbial membrane lipid structures are far more diverse than previously recognized5,6,7.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Grogan, D. W. et al. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol. Mol. Biol. Rev. 61, 446–449 (1997)
DeRosa, M. & Gambacorta, A. The lipids of archaebacteria. Prog. Lipid Res. 27, 153–175 (1988)
Wisotzkey, J. D., Jurtshuk, P. Jr, Fox, G. E., Deinhard, G. & Poralla, K. Comparative sequence analyses on the 16S rRNA (rDNA) of Bacillus acidocaldarius, Bacillus acidoterestris, and Bacillus cycloheptanicus and proposal for creation of a new genus, Alicyclobacillus gen. nov. Int. J. Syst. Bacteriol. 42, 263–269 (1992)
Li, W. & Fox, M. A. Syntheses, characterization, and photophysics studies of photoactive chromophore 2-naphthyl-labelled [n]-ladderanes. J. Am. Chem. Soc. 118, 11752–11758 (1996)
Schouten, S., Hopmans, E. C., Pancost, R. D. & Sinninghe Damsté, J. S. Widespread occurrence of structurally diverse tetraether membrane lipids: Evidence for the ubiquitous presence of low-temperature relatives of hyperthermophiles. Proc. Natl Acad. Sci. USA 97, 14421–14426 (2000)
Pancost, R. D., Bouloubassi, I., Aloisi, V., Sinninghe Damsté, J. S. & the MEDINAUT Shipboard Scientific Party Three series of non-isoprenoidal dialkyl glycerol diethers in cold-seep carbonate crusts. Org. Geochem. 32, 695–707 (2001)
Sinninghe Damsté, J. S., Hopmans, E. C., Pancost, R. D., Schouten, S. & Geenevasen, J. A. J. Newly discovered non-isoprenoid dialkyl diglycerol tetraether lipids in sediments. J. Chem. Soc. Chem. Commun. 1683–1684 (2000)
Strous, M. et al. Missing lithotroph identified as new planctomycete. Nature 400, 446–449 (1999)
Schmid, M. et al. Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Syst. Appl. Microbiol. 23, 93–106 (2000)
Egli, K. et al. Enrichment and characterization of an anammox bacterium from a rotating biological contactor treating ammonium-rich leachate. Arch. Microbiol. 175, 198–207 (2001)
Lindsay, M. R. et al. Cell compartmentalisation in planctomycetes: novel types of structural organisation for the bacterial cell. Arch. Microbiol. 175, 413–429 (2001)
van de Graaf, A. A., de Bruijn, P., Robertson, L. A., Jetten, M. S. M. & Kuenen, J. G. Metabolic pathway of anaerobic ammonium oxidation on basis of 15N-studies in a fluidized bed reactor. Microbiology 143, 2415–2421 (1997)
Olsen, G. J. What's eating the free lunch? Nature 400, 403–404 (1999)
Miller, M. A. & Schulman, J. M. AM1, MNDO and MM2 studies on concatenated cyclobutanes: Prismans, ladderanes and asteranes. J. Mol. Struct. 163, 133–141 (1988)
Sittig, M. & Schlesner, H. Chemotaxonomic investigation of various prosthecate and/or budding bacteria. Syst. Appl. Microbiol. 16, 92–103 (1993)
Van de Graaf, A. A. et al. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 142, 2187–2196 (1996)
Langworthy, T. A. & Pond, J. L. Archaebacterial ether lipids and chemotaxonomy. Syst. Appl. Microbiol. 7, 253–257 (1986)
Langworthy, T. A., Holzer, G., Zeikus, J. G. & Tornabene, T. G. Iso- and anteiso-branched glycerol diethers of the thermophilic anaerobe Thermodesulfobacterium commune. System. Appl. Microbiol. 4, 1–17 (1983)
Huber, R. et al. Formation of ammonium from nitrate during chemolithoautotrophic growth of the extremely thermophilic bacterium Ammonifex degensii gen. nov. sp. nov. Syst. Appl. Microbiol. 19, 40–49 (1996)
Huber, R. et al. Aquifex pyrophilus, new genus new species, represents a novel group of marine hyperthermophilic hydrogen-oxidizing bacteria. Syst. Appl. Microbiol. 15, 340–351 (1992)
DeRosa, M., et al. Microbiology of Extreme Environments and its Potential for Biotechnology (eds Da Costa, M. S., Duarte, J. C. & Williams, R. A. D.) 167–173 (Elsevier, London, 1989)
Van den Vossenberg, J. C. M., Driessen, A. J. M. & Konings, W. N. The essence of being extremophilic: the role of the unique archaeal membrane lipids. Extremophiles 2, 163–170 (1998)
Rütters, H., Sass, H., Cypionka, H. & Rullkötter, J. Monoalkylether phospholipids in the sulfate-reducing bacteria Desulfosarcina variabilis and Desulforhabdus amnigenus. Arch. Microbiol. 176, 435–442 (2001)
Mehta, G. et al. Quest for higher ladderanes: Oligomerization of a cyclobutadiene derivative. Angew. Chem. Int. Ed. 31, 1488–1490 (1992)
Strous, M., Heijnen, J. J., Kuenen, J. G. & Jetten, M. S. M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 50, 589–596 (1998)
Thamdrup, B. & Dalsgaard, T. Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl. Environ. Microbiol. 68, 1312–1318 (2002)
Schalk, J., de Vries, S., Kuenen, J. G. & Jetten, M. S. M. Involvement of a novel hydroxylamine oxidoreductase in anaerobic ammonium oxidation. Biochemistry 39, 5405–5412 (2000)
van Duin, A. C. T. & Larter, S. A computational chemical study of penetration and displacement of water films near mineral surfaces. Geochem. Trans. 006 (2001)
Berendsen, H. J. C., Postma, J. P. M., van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984)
Acknowledgements
We thank J. G. Kuenen, H. Hiemstra, S. Schouten and W. Konings for stimulating discussions, C. Erkelens (University of Leiden) for access to the 600- and 750-MHz NMR instruments, J. A. Fuerst for cells of Gemmata obscuriglobus and Pirellula sp. and training of L.A.v.N., M. Wolters-Arts for help with electron microscopy, and K. T. van de Pas-Schoonen for help with immunofluorescence.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Sinninghe Damsté, J., Strous, M., Rijpstra, W. et al. Linearly concatenated cyclobutane lipids form a dense bacterial membrane. Nature 419, 708–712 (2002). https://doi.org/10.1038/nature01128
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01128
This article is cited by
-
Mining genomes to illuminate the specialized chemistry of life
Nature Reviews Genetics (2021)
-
Bridging the membrane lipid divide: bacteria of the FCB group superphylum have the potential to synthesize archaeal ether lipids
The ISME Journal (2021)
-
Formation and function of bacterial organelles
Nature Reviews Microbiology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.