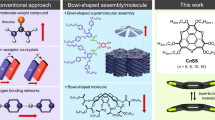
Abstract
Polar liquid crystalline materials can be used in optical and electronic applications, and recent interest has turned to formation strategies that exploit the shape of polar molecules and their interactions to direct molecular alignment1,2. For example, banana-shaped molecules align their molecular bent within smectic layers3, whereas conical molecules should form polar columnar assemblies4,5,6,7,8,9. However, the flatness of the conical molecules used until now4,5,6,9 and their ability to flip7,8 have limited the success of this approach to making polar liquid crystalline materials. Here we show that the attachment of five aromatic groups to one pentagon of a C60 fullerene molecule yields deeply conical molecules that stack into polar columnar assemblies. The stacking is driven by attractive interactions between the spherical fullerene moiety and the hollow cone formed by the five aromatic side groups of a neighbouring molecule in the same column. This packing pattern is maintained when we extend the aromatic groups by attaching flexible aliphatic chains, which yields compounds with thermotropic and lyotropic liquid crystalline properties. In contrast, the previously reported fullerene-containing liquid crystals10,11,12,13,14,15,16,17 all exhibit thermotropic properties only, and none of them contains the fullerene moiety as a functional part of its mesogen units. Our design strategy should be applicable to other molecules and yield a range of new polar liquid crystalline materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Guillon, D. Molecular engineering for ferroelectricity in liquid crystals. Adv. Chem. Phys. 113, 1–49 (2000)
Kato, T. Self-assembly of phase-segregated liquid crystal structures. Science 295, 2414–2418 (2002)
Niori, T., Sekine, T., Watanabe, J., Furukawa, T. & Takezoe, H. Distinct ferroelectric smectic liquid crystals consisting of banana shaped achiral molecules. J. Mater. Chem. 6, 1231–1233 (1996)
Cometti, G., Dalcanale, E., Du Vosel, A. & Levelut, A.-M. New bowl-shaped columnar liquid crystals. J. Chem. Soc. Chem. Commun. 163–165 (1990)
Xu, B. & Swager, T. M. Rigid bowlic liquid crystals based on tungsten-oxo calix[4]arenes: host-guest effects and head-to-tail organization. J. Am. Chem. Soc. 115, 1159–1160 (1993)
Komori, T. & Shinkai, S. Novel columnar liquid crystals designed from cone-shaped calix[4]arenes. The rigid bowl is essential for the formation of the liquid crystal phase. Chem. Lett. 1455–1458 (1993)
Malthête, J. & Collet, A. Liquid crystals with a cone-shaped cyclotriveratrylene core. Nouv. J. Chim. 9, 151–153 (1985)
Malthête, J. & Collet, A. Inversion of the cyclotribenzylene cone in a columnar mesophase: a potential way to ferroelectric materials. J. Am. Chem. Soc. 109, 7544–7545 (1987)
Kang, S. H. et al. Novel columnar mesogen with octupolar optical nonlinearities: synthesis, mesogenic behaviour and multiphoton-fluorescence-free hyperpolarizabilities of subphthalocyanines with long aliphatic chains. Chem. Commun. 1661–1662 (1999)
Chuard, T. & Deschenaux, R. First fullerene[60]-containing thermotropic liquid crystal. Helv. Chim. Acta 79, 736–741 (1996)
Chuard, T., Deschenaux, R., Hirsch, A. & Schönberger, H. A liquid-crystalline hexa-adduct of [60]fullerene. Chem. Commun. 2103–2104 (1999)
Tirelli, N., Cardullo, F., Habicher, T., Suter, U. W. & Diederich, F. Thermotropic behaviour of covalent fullerene adducts displaying 4-cyano-4'-oxybiphenyl mesogens. J. Chem. Soc. Perkin Trans. 2, 193–198 (2000)
Felder, D., Heinrich, B., Guillon, D., Nicoud, J.-F. & Nierengarten, J.-F. A liquid crystalline supramolecular complex of C60 with a cyclotriveratrylene derivative. Chem. Eur. J. 6, 3501–3507 (2000)
Dardel, B., Guillon, D., Heinrich, B. & Deschenaux, R. Fullerene-containing liquid-crystalline dendrimers. J. Mater. Chem. 11, 2814–2831 (2001)
Chuard, T. & Deschenaux, R. Design, mesomorphic properties, and supramolecular organization of [60]fullerene-containing thermotropic liquid crystals. J. Mater. Chem. 12, 1944–1951 (2002)
Suzuki, M., Furue, H. & Kobayashi, S. Polarizerless nanomaterial doped guest-host LCD exhibiting high luminance and good legibility. Mol. Cryst. Liq. Cryst. 368, 191–196 (2001)
Kimura, M. et al. Self-organization of supramolecular complex composed of rigid dendritic porphyrin and fullerene. J. Am. Chem. Soc. 124, 5274–5275 (2002)
Georgakilas, V. et al. Supramolecular self-asssembled fullerene nanostructures. Proc. Natl Acad. Sci. 99, 5075–5080 (2002)
Sawamura, M., Iikura, H. & Nakamura, E. The first pentahaptofullerene metal complexes. J. Am. Chem. Soc. 118, 12850–12851 (1996)
Tschierske, C. Non-conventional liquid crystals-the importance of micro-segregation for self-organization. J. Mater. Chem. 8, 1485–1508 (1998)
Ruoff, R. S., Tse, D. S., Malhotra, R. & Lorents, D. C. Solubility of C60 in a variety of solvents. J. Phys. Chem. 97, 3379–3383 (1993)
Iikura, H., Mori, S., Sawamura, M. & Nakamura, E. Endohedral homoconjugation in cyclopentadiene embedded in C60. Theoretical and electrochemical evidence. J. Org. Chem. 62, 7912–7913 (1997)
Sawamura, M., Kuninobu, Y. & Nakamura, E. Half-sandwich metallocene embedded in a spherically extended π-conjugate system. Synthesis, structure, and electrochemistry of Rh(η5-C60Me5)(CO)2 . J. Am. Chem. Soc. 122, 12407–12408 (2000)
Sawamura, M. et al. Hybrid of ferrocene and fullerene. J. Am. Chem. Soc. 124, 9354–9355 (2002)
Manners, I. Putting metals into polymers. Science 294, 1664–1666 (2001)
Acknowledgements
We thank Frontier Carbon Corporation for generous supply of C60. The present research was supported by a Grant-in-Aid for Scientific Research (Specially Promoted Research) from the Ministry of Education, Culture, Sports, Science, and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Sawamura, M., Kawai, K., Matsuo, Y. et al. Stacking of conical molecules with a fullerene apex into polar columns in crystals and liquid crystals. Nature 419, 702–705 (2002). https://doi.org/10.1038/nature01110
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01110
This article is cited by
-
Enhancing physical characteristics of thermotropic nematic liquid crystals by dispersing in various nanoparticles and their potential applications
Emergent Materials (2023)
-
Functional liquid-crystalline polymers and supramolecular liquid crystals
Polymer Journal (2018)
-
Antiviral activity of fullerene C60 nanocrystals modified with derivatives of anionic antimicrobial peptide maximin H5
Monatshefte für Chemie - Chemical Monthly (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.