Abstract
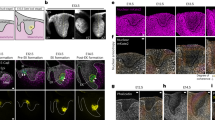
Hearing sensitivity in mammals is enhanced by more than 40 dB (that is, 100-fold) by mechanical amplification thought to be generated by one class of cochlear sensory cells, the outer hair cells1,2,3,4. In addition to the mechano-electrical transduction required for auditory sensation, mammalian outer hair cells also perform electromechanical transduction, whereby transmembrane voltage drives cellular length changes at audio frequencies in vitro5,6,7. This electromotility is thought to arise through voltage-gated conformational changes in a membrane protein8,9, and prestin has been proposed as this molecular motor10,11,12. Here we show that targeted deletion of prestin in mice results in loss of outer hair cell electromotility in vitro and a 40–60 dB loss of cochlear sensitivity in vivo, without disruption of mechano-electrical transduction in outer hair cells. In heterozygotes, electromotility is halved and there is a twofold (about 6 dB) increase in cochlear thresholds. These results suggest that prestin is indeed the motor protein, that there is a simple and direct coupling between electromotility and cochlear amplification, and that there is no need to invoke additional active processes to explain cochlear sensitivity in the mammalian ear.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gold, T. Hearing. II. The physical basis of the action of the cochlea. Proc. R. Soc. Lond. B 135, 492–498 (1948)
Dallos, P. & Harris, D. Properties of auditory nerve responses in absence of outer hair cells. J. Neurophysiol. 41, 365–383 (1978)
Brown, M. C., Nuttall, A. L. & Masta, R. I. Intracellular recordings from cochlear inner hair cells: effects of stimulation of the crossed olivocochlear efferents. Science 222, 69–72 (1983)
Dallos, P. The active cochlea. J. Neurosci. 12, 4575–4585 (1992)
Brownell, W. E., Bader, C. R., Bertrand, D. & de Ribaupierre, Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science 227, 194–196 (1985)
Kachar, B., Brownell, W. E., Altschuler, R. & Fex, J. Electrokinetic shape changes of cochlear outer hair cells. Nature 322, 365–368 (1986)
Ashmore, J. F. A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J. Physiol. 388, 323–347 (1987)
Ashmore, J. F. Cochlear Mechanisms (eds Wilson, J. P. & Kemp, D. T.) 107–116 (Plenum, London, 1989)
Santos-Sacchi, J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J. Neurosci. 11, 3096–3110 (1991)
Zheng, J. et al. Prestin is the motor protein of cochlear outer hair cells. Nature 405, 149–155 (2000)
Belyantseva, I. A., Adler, H. J., Curi, R., Frolenkov, G. I. & Kachar, B. Expression and localization of prestin and the sugar transporter GLUT-5 during development of electromotility in cochlear outer hair cells. J. Neurosci. 20, RC116 (2000)
Oliver, D. et al. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science 292, 2340–2343 (2001)
Forge, A. Structural features of the lateral walls in mammalian cochlear outer hair cells. Cell Tissue Res. 265, 473–483 (1991)
Dallos, P. & Fakler, B. Prestin, a new type of motor protein. Nature Rev. Mol. Cell Biol. 3, 104–111 (2002)
Bohne, B. A. & Rabbitt, K. D. Holes in the reticular lamina after noise exposure: implication for continuing damage in the organ of Corti. Hear. Res. 11, 41–53 (1983)
Holt, J. R. et al. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell 108, 371–381 (2002)
Kros, C. J. et al. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nature Neurosci. 5, 41–47 (2002)
Dallos, P. & Wang, C. Y. Bioelectric correlates of kanamycin intoxication. Audiology 13, 277–289 (1974)
Kemp, D. T. Stimulated acoustic emissions from within the human auditory system. J. Acoust. Soc. Am. 64, 1386–1391 (1978)
Johnstone, B. M., Patuzzi, R. & Yates, G. K. Basilar membrane measurements and the travelling wave. Hear. Res. 22, 147–153 (1986)
Ruggero, M. A. & Rich, N. C. Application of a commercially-manufactured Doppler-shift laser velocimeter to the measurement of basilar-membrane vibration. Hear. Res. 51, 215–230 (1991)
Sellick, P. M., Patuzzi, R. & Johnstone, B. M. Measurement of basilar membrane motion in the guinea pig using the Mossbauer technique. J. Acoust. Soc. Am. 72, 131–141 (1982)
Kiang, N. Y. & Moxon, E. C. Tails of tuning curves of auditory-nerve fibers. J. Acoust. Soc. Am. 55, 620–630 (1974)
Ruggero, M. A. Responses to sound of the basilar membrane of the mammalian cochlea. Curr. Opin. Neurobiol. 2, 449–456 (1992)
Manley, G. A. Cochlear mechanisms from a phylogenetic viewpoint. Proc. Natl Acad. Sci. USA 97, 11736–11743 (2000)
Fettiplace, R., Ricci, A. J. & Hackney, C. M. Clues to the cochlear amplifier from the turtle ear. Trends Neurosci. 24, 169–175 (2001)
Hudspeth, A. J. Mechanical amplification of stimuli by hair cells. Curr. Opin. Neurobiol. 7, 480–486 (1997)
Ehret, G. The Auditory Psychobiology of the Mouse (ed. Willott, J. F.)) 169–200 (Charles Thomas, Springfield, Illinois, 1983)
He, D. Z., Evans, B. N. & Dallos, P. First appearance and development of electromotility in neonatal gerbil outer hair cells. Hear. Res. 78, 77–90 (1994)
Acknowledgements
We thank K. Cullen for technical assistance; T. Curran, B. Fritzsch, C. A. Shera and D. Freeman for comments on the manuscript; and B. Kachar, T. Hasson and P. Gillespie for antibodies. This work is supported in part by NIH grants to M.C.L., Z.Z.H. and J.Z., NIH Cancer Center Support CORE grant, and the American Lebanese Syrian Associated Charities (ALSAC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Liberman, M., Gao, J., He, D. et al. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 419, 300–304 (2002). https://doi.org/10.1038/nature01059
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature01059
This article is cited by
-
Intratympanic drug delivery systems to treat inner ear impairments
Journal of Pharmaceutical Investigation (2023)
-
Evaluation of the relationship between prestin serum biomarker and sensorineural hearing loss: a case–control study
European Archives of Oto-Rhino-Laryngology (2023)
-
Compression in the Auditory System
Neuroscience and Behavioral Physiology (2023)
-
The Long Outer-Hair-Cell RC Time Constant: A Feature, Not a Bug, of the Mammalian Cochlea
Journal of the Association for Research in Otolaryngology (2023)
-
Fgf8P2A-3×GFP/+: A New Genetic Mouse Model for Specifically Labeling and Sorting Cochlear Inner Hair Cells
Neuroscience Bulletin (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.