Abstract
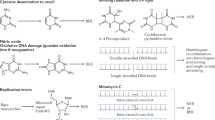
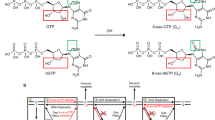
The bacterial AlkB protein is known to be involved in cellular recovery from alkylation damage; however, the function of this protein remains unknown. AlkB homologues have been identified in several organisms, including humans, and a recent sequence alignment study has suggested that these proteins may belong to a superfamily of 2-oxoglutarate-dependent and iron-dependent oxygenases (2OG-Fe(ii)-oxygenases)1. Here we show that AlkB from Escherichia coli is indeed a 2-oxoglutarate-dependent and iron-dependent DNA repair enzyme that releases replication blocks in alkylated DNA by a mechanism involving oxidative demethylation of 1-methyladenine residues. This mechanism represents a new pathway for DNA repair and the third type of DNA damage reversal mechanism so far discovered.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Aravind, L. & Koonin, E. V. The DNA-repair protein AlkB, EGL-9, and leprecan define new families of 2-oxoglutarate- and iron-dependent dioxygenases. Genome Biol. 2, 0007.1–0007.8 (2001)
Pegg, A. E. Repair of O(6)-alkylguanine by alkyltransferases. Mutat. Res. 462, 83–100 (2000)
Evensen, G. & Seeberg, E. Adaptation to alkylation resistance involves the induction of a DNA glycosylases. Nature 296, 773–775 (1982)
Karran, P., Hjelmgren, T. & Lindahl, T. Induction of a DNA glycosylase for N-methylated purines is part of the adaptive response to alkylating agents. Nature 296, 770–773 (1982)
Karran, P., Lindahl, T. & Griffin, B. Adaptive response to alkylating agents involves alternation in situ of O6-methylguanine residues in DNA. Nature 280, 76–77 (1979)
Kataoka, H., Yamamoto, Y. & Sekiguchi, M. A new gene (alkB) of Escherichia coli that controls sensitivity to methyl methane sulfonate. J. Bacteriol. 153, 1301–1307 (1983)
Wei, Y. F., Carter, K. C., Wang, R. P. & Shell, B. K. Molecular cloning and functional analysis of a human cDNA encoding an Escherichia coli AlkB homolog, a protein involved in DNA alkylation damage repair. Nucleic Acids Res. 24, 931–937 (1996)
Dinglay, S., Trewick, S. C., Lindahl, T. & Sedgwick, B. Defective processing of methylated single-stranded DNA by E. coli AlkB mutants. Genes Dev. 14, 2097–2105 (2000)
Parkinson, A. Casarett and Doull's Toxicology (ed. Claassen, C. D.) 113–186 (McGraw-Hill, New York, 1996)
Nash, T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem. J. 55, 416–421 (1953)
Rapoport, R., Hanukoglu, I. & Sklan, D. A fluorimetric assay for hydrogen peroxide, suitable for NAD(P)H-dependent superoxide generating redox systems. Anal. Biochem. 218, 309–313 (1994)
Chen, B. J., Carroll, P. & Samson, L. The Escherichia coli AlkB protein protects human cells against alkylation-induced toxicity. J. Bacteriol. 176, 6255–6261 (1994)
Singer, B. & Grunberger, D. Molecular Biology of Mutagens and Carcinogens (Plenum, New York, 1983)
Amersham Pharmacia Biotech GST Gene Fusion System: User Manual; (1997) 〈www.amershambiosciences.com〉
Bjelland, S., Bjørås, M. & Seeberg, E. Excision of 3-methylguanine from alkylated DNA by 3-methyladenine DNA glycosylase I of Escherichia coli. Nucleic Acids Res. 21, 2045–2049 (1993)
Wang, G., Rahman, M. S. & Humayun, M. Z. Replication of M13 single-stranded viral DNA bearing single site-specific adducts by Escherichia coli cell extracts: differential efficiency of translesion DNA synthesis for SOS-dependent and SOS-independent lesions. Biochemistry 36, 9486–9492 (1997)
Palejwala, V. A., Simha, D. & Humayun, M. Z. Mechanisms of mutagenesis by exocyclic DNA adducts. Transfection of M13 viral DNA bearing a site-specific adduct shows that ethenocytosine is a highly efficient RecA-independent mutagenic noninstructional lesion. Biochemistry 30, 8736–8743 (1991)
Chung, C. T., Niemela, S. L. & Miller, R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc. Natl Acad. Sci. USA 86, 2172–2175 (1989)
Hofer, T. & Moller, L. Reduction of oxidation during the preparation of DNA and analysis of 8-hydroxy-2′-deoxyguanosine. Chem. Res. Toxicol. 11, 882–887 (1998)
Acknowledgements
We thank M. Bjørås, L. Eide, K. Baynton, K. I. Kristiansen, J. Myllyharju, K. Skarstad and J. Klaveness for help and discussions, and L. Eide, K. Baynton and A. Klungland for critical reading of the manuscript. We are grateful to M. Bjørås for preparation of [3H]MNU-labelled DNA substrates, to L. Eide for the construction of the plasmid for expression of GST–AlkB, and to D. Daoudi for technical assistance. This work was supported by the Research Council of Norway and the Norwegian Cancer Society. E.S. also acknowledges support from the European Commission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Falnes, P., Johansen, R. & Seeberg, E. AlkB-mediated oxidative demethylation reverses DNA damage in Escherichia coli. Nature 419, 178–182 (2002). https://doi.org/10.1038/nature01048
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01048
This article is cited by
-
Spatial and single-cell analyses uncover links between ALKBH1 and tumor-associated macrophages in gastric cancer
Cancer Cell International (2024)
-
A DNA adenine demethylase impairs PRC2-mediated repression of genes marked by a specific chromatin signature
Genome Biology (2023)
-
Unlocking the secrets: the power of methylation-based cfDNA detection of tissue damage in organ systems
Clinical Epigenetics (2023)
-
Signaling pathways in brain tumors and therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
Pangenomic Analysis of Nucleo-Cytoplasmic Large DNA Viruses. I: The Phylogenetic Distribution of Conserved Oxygen-Dependent Enzymes Reveals a Capture-Gene Process
Journal of Molecular Evolution (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.