Abstract
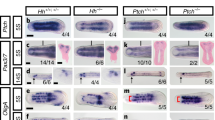
Arthropods and higher vertebrates both possess appendages, but these are morphologically distinct and the molecular mechanisms regulating patterning along their proximodistal axis (base to tip) are thought to be quite different. In Drosophila, gene expression along this axis is thought to be controlled primarily by a combination of transforming growth factor-β (TGF-ß) and Wnt signalling from sources of ligands, Decapentaplegic (Dpp) and Wingless (Wg), in dorsal and ventral stripes, respectively1,2,3. In vertebrates, however, proximodistal patterning is regulated by receptor tyrosine kinase (RTK) activity from a source of ligands, fibroblast growth factors (FGFs), at the tip of the limb bud4. Here I revise our understanding of limb development in flies and show that the distal region is actually patterned by a distal-to-proximal gradient of RTK activity, established by a source of epidermal growth factor (EGF)-related ligands at the presumptive tip. This similarity between proximodistal patterning in vertebrates and flies supports previous suggestions5,6 of an evolutionary relationship between appendages/body-wall outgrowths in animals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Campbell, G., Weaver, T. & Tomlinson, A. Axis specification in the developing Drosophila appendage: the role of wingless, decapentaplegic, and the homeobox gene aristaless. Cell 74, 1113–1123 (1993)
Diaz-Benjumea, F. J., Cohen, B. & Cohen, S. M. Cell interaction between compartments establishes the proximal-distal axis of Drosophila legs. Nature 372, 175–179 (1994)
Lecuit, T. & Cohen, S. M. Proximal-distal axis formation in the Drosophila leg. Nature 288, 139–145 (1997)
Martin, G. R. The roles of FGFs in the early development of vertebrate limbs. Genes Dev. 12, 1571–1586 (1998)
Panganiban, G. et al. The origin and evolution of animal appendages. Proc. Natl Acad. Sci. USA 94, 5162–5166 (1997)
Shubin, N., Tabin, C. & Carroll, S. Fossils, genes and the evolution of animal limbs. Nature 388, 639–648 (1997)
Campbell, G. & Tomlinson, A. Initiation of the proximodistal axis in insect legs. Development 121, 619–628 (1995)
Casares, F. & Mann, R. S. The ground state of the ventral appendage in Drosophila. Science 293, 1477–1480 (2001)
Wehrli, M. et al. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407, 527–530 (2000)
Nellen, D., Affolter, M. & Basler, K. Receptor serine/threonine kinases implicated in the control of Drosophila body pattern by decapentaplegic. Cell 78, 225–237 (1994)
Penton, A. et al. Identification of two bone morphogenetic protein type I receptors in Drosophila and evidence that Brk25D is a decapentaplegic receptor. Cell 78, 239–250 (1994)
Clifford, R. J. & Schupbach, T. Coordinately and differentially mutable activities of torpedo, the Drosophila melanogaster homolog of the vertebrate EGF receptor gene. Genetics 123, 771–787 (1989)
Campbell, G. & Tomlinson, A. The roles of the homeobox genes aristaless and Distal-less in patterning the legs and wings of Drosophila. Development 125, 4483–4493 (1998)
Kumar, J. P. et al. Dissecting the roles of the Drosophila EGF receptor in eye development and MAP kinase activation. Development 125, 3875–3885 (1998)
Halfar, K., Rommel, C., Stocker, H. & Hafen, E. Ras controls growth, survival and differentiation in the Drosophila eye by different thresholds of MAP kinase activity. Development 128, 1687–1696 (2001)
Kojima, T., Sato, M. & Saigo, K. Formation and specification of distal leg segments in Drosophila by dual Bar homeobox genes, BarH1 and BarH2. Development 127, 769–778 (2000)
Couso, J. P. & Bishop, S. A. Proximo-distal development in the legs of Drosophila. Int. J. Dev. Biol. 42, 345–352 (1998)
Schweitzer, R. & Shilo, B. Z. A thousand and one roles for the Drosophila EGF receptor. Trends Genet. 13, 191–196 (1997)
Bang, A. G. & Kintner, C. Rhomboid and Star facilitate presentation and processing of the Drosophila TGF-α homolog Spitz. Genes Dev. 14, 177–186 (2000)
Wasserman, J. D., Urban, S. & Freeman, M. A family of rhomboid-like genes: Drosophila rhomboid-1 and roughoid/rhomboid-3 cooperate to activate EGF receptor signaling. Genes Dev. 14, 1651–1663 (2000)
Kramer, S., Okabe, M., Hacohen, N., Krasnow, M. A. & Hiromi, Y. Sprouty: a common antagonist of FGF and EGF signaling pathways in Drosophila. Development 126, 2515–2525 (1999)
Casci, T., Vinos, J. & Freeman, M. Sprouty, an intracellular inhibitor of Ras signaling. Cell 96, 655–665 (1999)
Irvine, K. D. Fringe Notch, and making developmental boundaries. Curr. Opin. Genet. Dev. 9, 434–441 (1999)
Queenan, A. M., Ghabrial, A. & Schupbach, T. Ectopic activation of torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development 124, 3871–3880 (1997)
Brook, W. J. & Cohen, S. M. Antagonistic interactions between wingless and decapentaplegic responsible for dorsal-ventral pattern in the Drosophila leg. Science 273, 1373–1377 (1996)
Mardon, G., Solomon, N. M. & Rubin, G. M. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development 120, 3473–3486 (1994)
Struhl, G. & Basler, K. Organizing activity of wingless protein in Drosophila. Cell 72, 527–540 (1993)
Acknowledgements
I thank A. Tomlinson, M. Simcox, D. Chapman and R. Carthew for advice and comments on the manuscript. I also thank the following for stocks and reagents: K. Moses, J. Kumar, M. Werhli, A. Simcox, J. P. Couso, S. Kunes, T. Schubach, T. Volk, G. Struhl, H. M. Chung, M. Feeman, R. Cagan, T. Kojima, S. Cohen, K. Cook, K. Matthews, the Bloomington Stock Center, J. P. Vincent, and the Developmental Studies Hybridoma Bank.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares that he has no competing financial interests.
Rights and permissions
About this article
Cite this article
Campbell, G. Distalization of the Drosophila leg by graded EGF-receptor activity. Nature 418, 781–785 (2002). https://doi.org/10.1038/nature00971
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature00971
This article is cited by
-
aristaless1 has a dual role in appendage formation and wing color specification during butterfly development
BMC Biology (2023)
-
Egfr signaling promotes juvenile hormone biosynthesis in the German cockroach
BMC Biology (2022)
-
Activation of EGFR signaling by Tc-Vein and Tc-Spitz regulates the metamorphic transition in the red flour beetle Tribolium castaneum
Scientific Reports (2021)
-
Hedgehog signaling regulates regenerative patterning and growth in Harmonia axyridis leg
Cellular and Molecular Life Sciences (2021)
-
The pivotal role of aristaless in development and evolution of diverse antennal morphologies in moths and butterflies
BMC Evolutionary Biology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.