Abstract
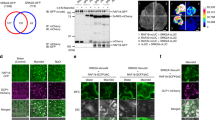
Protein kinases are involved in stress signalling in both plant and animal systems. The hormone abscisic acid mediates the responses of plants to stresses such as drought, salinity and cold. Abscisic-acid-activated protein kinase (AAPK)—found in guard cells, which control stomatal pores—has been shown to regulate plasma membrane ion channels1. Here we show that AAPK-interacting protein 1 (AKIP1), with sequence homology to heterogeneous nuclear RNA-binding protein A/B, is a substrate of AAPK. AAPK-dependent phosphorylation is required for the interaction of AKIP1 with messenger RNA that encodes dehydrin, a protein implicated in cell protection under stress conditions. AAPK and AKIP1 are present in the guard-cell nucleus, and in vivo treatment of such cells with abscisic acid enhances the partitioning of AKIP1 into subnuclear foci which are reminiscent of nuclear speckles. These results show that phosphorylation-regulated RNA target discrimination by heterogeneous nuclear RNA-binding proteins2 may be a general phenomenon in eukaryotes, and implicate a plant hormone in the regulation of protein dynamics during rapid subnuclear reorganization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Li, J., Wang, X. Q., Watson, M. B. & Assmann, S. M. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science 287, 300–303 (2000)
Ostrowski, J. et al. Insulin alters heterogeneous nuclear ribonucleoprotein K protein binding to DNA and RNA. Proc. Natl Acad. Sci. USA 98, 9044–9049 (2001)
Schroeder, J. I., Kwak, J. M. & Allen, G. J. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 410, 327–330 (2001)
Assmann, S. M. & Wang, X.-Q. From milliseconds to millions of years: guard cells and environmental responses. Curr. Opin. Plant Biol. 4, 421–428 (2001)
Burd, C. G. & Dreyfuss, G. Conserved structures and diversity of functions of RNA-binding proteins. Science 265, 615–621 (1994)
Krecic, A. M. & Swanson, M. S. hnRNP complexes: composition, structure, and function. Curr. Opin. Cell Biol. 11, 363–371 (1999)
Khan, F. A., Jaiswal, A. K. & Szer, W. Cloning and sequence analysis of a human type A/B hnRNP protein. FEBS Lett. 290, 159–161 (1991)
Misteli, T. Protein dynamics: implications for nuclear architecture and gene expression. Science 291, 843–847 (2001)
Li, J. & Assmann, S. M. An ABA-activated and calcium-independent protein kinase from guard cells of fava bean. Plant Cell 8, 2359–2368 (1996)
Mori, I. C. & Muto, S. Abscisic acid activates a 48-kilodalton protein kinase in guard cell protoplasts. Plant Physiol. 113, 833–839 (1997)
Busk, P. K. & Pages, M. Regulation of abscisic acid-induced transcription. Plant Mol. Biol. 37, 425–435 (1998)
Shen, L., Outlaw, W. H. Jr & Epstein, L. M. Expression of an mRNA with sequence similarity to pea dehydrin (Psdhn 1) in guard cells of Vicia faba in response to exogenous abscisic acid. Physiol. Planta 95, 99–105 (1995)
Dixon, A. K., Richardson, P. J., Lee, K., Carter, N. P. & Freeman, T. C. Expression profiling of single cells using 3 prime end amplification (TPEA) PCR. Nucleic Acids Res. 26, 4426–4431 (1998)
Li, J., Lee, Y. R. & Assmann, S. M. Guard cells possess a calcium-dependent protein kinase that phosphorylates the KAT1 potassium channel. Plant Physiol. 116, 785–795 (1998)
Freire, M. A. & Pagès, M. Functional characteristics of the maize RNA-binding protein MA16. Plant Mol. Biol. 29, 797–807 (1995)
Lorkovic, Z. J. & Barta, A. Genome analysis: RNA recognition motif (RRM) and K homology (KH) domain RNA-binding proteins from the flowering plant Arabidopsis thaliana. Nucleic Acids Res. 30, 623–635 (2002)
Lorkovic, Z. J., Wieczorek Kirk, D. A., Lambermon, M. H. L. & Filipowicz, W. Pre-mRNA splicing in higher plants. Trends Plant Sci. 5, 160–167 (2000)
Lambermon, M. H. L. et al. UBP1, a novel hnRNP-like protein that functions at multiple steps of higher plant nuclear pre-mRNA maturation. EMBO J. 19, 1638–1649 (2000)
Macknight, R. et al. FCA, a gene controlling flowering time in Arabidopsis, encodes a protein containing RNA-binding domains. Cell 89, 737–745 (1997)
Schomburg, F. M., Patton, D. A., Meinke, D. W. & Amasino, R. M. FPA, a gene involved in floral induction in Arabidopsis, encodes a protein containing RNA-recognition motifs. Plant Cell 13, 1427–1436 (2001)
Lu, C. & Fedoroff, N. A mutation in the Arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell 12, 2351–2366 (2000)
Xiong, L. et al. Modulation of abscisic acid signal transduction and biosynthesis by an Sm-like protein in Arabidopsis. Dev. Cell 1, 771–781 (2001)
Hugouvieux, V., Kwak, J. M. & Schroeder, J. I. An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell 106, 477–487 (2001)
Gómez, J. et al. A gene induced by the plant hormone abscisic acid in response to water stress encodes a glycine-rich protein. Nature 344, 262–264 (1988)
Dunn, M. A., Brown, K., Lightowlers, R. & Hughes, M. A. A low-temperature-responsive gene from barley encodes a protein with single-stranded nucleic acid-binding activity which is phosphorylated in vitro. Plant Mol. Biol. 30, 947–959 (1996)
Melcak, I., Melcakova, S., Kopsky, V., Vecerova, J. & Raska, I. Prespliceosomal assembly on microinjected precursor mRNA takes place in nuclear speckles. Mol. Biol. Cell 12, 393–406 (2001)
Stone, J. M., Collinge, M. A., Smith, R. D., Horn, M. A. & Walker, J. C. Interaction of a protein phosphatase with an Arabidopsis serine-threonine receptor kinase. Science 266, 793–795 (1994)
Gygi, S. P., Rochon, Y., Franza, B. R. & Aebersold, R. Correlation between protein and mRNA abundance in yeast. Mol. Cell. Biol. 19, 1720–1730 (1999)
Kinoshita, T. & Shimazaki, K. Blue light activates the plasma membrane H+-ATPase by phosphorylation of C-terminus in stomatal guard cells. EMBO J. 18, 5548–5558 (1999)
Moz, Y., Silver, J. & Naveh-Many, T. Protein-RNA interactions determine the stability of the renal NaPi-2 cotransporter mRNA and its translation in hypophosphatemic rats. J. Biol. Chem. 274, 25266–25272 (1999)
Acknowledgements
We thank S. Gilroy and E. Kunze for the use of their confocal and fluorescence microscopes; M. Guiltinan for use of the gene gun; H. Ma for providing the SNF1 and SNF4 constructs; P. James for providing the PJ69-4A strain; L. Ding for assistance with AKIP1 constructs; and P. Minnich for technical support. This research was supported by NSF (S.M.A.) and partly supported by a Grant-in-Aid for Scientific Research on Priority Areas from Ministry of Education, Sports and Culture of Japan (K.S. and T.K.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, J., Kinoshita, T., Pandey, S. et al. Modulation of an RNA-binding protein by abscisic-acid-activated protein kinase. Nature 418, 793–797 (2002). https://doi.org/10.1038/nature00936
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature00936
This article is cited by
-
Genome-wide association analysis for drought tolerance and component traits in groundnut gene pool
Euphytica (2024)
-
Simultaneous augmentation of muscle and bone by locomomimetism through calcium-PGC-1α signaling
Bone Research (2022)
-
Genetic dissection of drought resistance based on root traits at the bud stage in common bean
Theoretical and Applied Genetics (2021)
-
Loss of microRNA-23–27–24 clusters in skeletal muscle is not influential in skeletal muscle development and exercise-induced muscle adaptation
Scientific Reports (2019)
-
The sucrose non-fermenting 1-related kinase 2 gene SAPK9 improves drought tolerance and grain yield in rice by modulating cellular osmotic potential, stomatal closure and stress-responsive gene expression
BMC Plant Biology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.