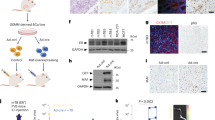
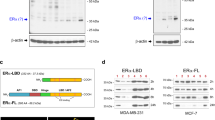
Abstract
Oestrogen receptor (ER) is a good prognostic marker for the treatment of breast cancers. Upregulation of metastatic tumour antigen 1 (MTA1) is associated with the invasiveness and metastatic potential of several human cancers1,2 and acts as a co-repressor of nuclear ER-α3. Here we identify a naturally occurring short form of MTA1 (MTA1s) that contains a previously unknown sequence of 33 amino acids with an ER-binding motif, Leu-Arg-Ile-Leu-Leu (LRILL). MTA1s localizes in the cytoplasm, sequesters ER in the cytoplasm, and enhances non-genomic responses of ER. Deleting the LRILL motif in MTA1s abolishes its co-repressor function and its interaction with ER, and restores nuclear localization of ER. Dysregulation of human epidermal growth factor receptor-2 in breast cancer cells enhances the expression of MTA1s and the cytoplasmic sequestration of ER. Expression of MTA1s in breast cancer cells prevents ligand-induced nuclear translocation of ER and stimulates malignant phenotypes. MTA1s expression is increased in human breast tumours with no or low nuclear ER. The regulation of the cellular localization of ER by MTA1s represents a mechanism for redirecting nuclear receptor signalling by nuclear exclusion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Toh, Y., Pencil, S. D. & Nicolson, G. L. A novel candidate metastasis-associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines. cDNA cloning, expression, and protein analyses. J. Biol. Chem. 269, 229–263 (1994)
Nawa, A. et al. Tumor metastasis-associated human MTA1 gene: its deduced protein sequence, localization and association with breast cancer cell proliferation using antisense phosphorothioate oligonucleotides. J. Cell. Biochem. 79, 202–212 (2000)
Mazumdar, A. et al. Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nature Cell Biol. 3, 30–37 (2001)
Shapiro, M. B. & Senapathy, P. RNA splices junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acid Res. 15, 7155–7174 (1987)
Crawford, J., Ianzano, L. & Savino, M. The PISSLRE gene: structure, exon skipping and exclusion as tumour suppressor in breast cancer. Genomics 56, 90–97 (1999)
Benz, C. C. et al. Estrogen-dependent, taxoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res. Treat. 24, 85–95 (1992)
Kumar, R. et al. Overexpression of HER2 modulates Bcl-2, Bcl-XL and Tamoxifen-induced apoptosis in human MCF-7 breast cancer cells. Clin. Cancer Res. 2, 1215–1219 (1996)
Vadlamudi, R. et al. PELP1, a novel human protein that acts as a bifunctional regulator of steroid receptors. J. Biol. Chem. 276, 38272–38279 (2001)
Henttu, P. M., Kalkhoven, E. & Parker, M. G. AF-2 activity and recruitment of steroid receptor coactivator 1 to the estrogen receptor depend on a lysine residue conserved in nuclear receptors. Mol. Cell. Biol. 17, 1832–1839 (1997)
Kumar, V. et al. Functional domains of the human estrogen receptor. Cell 51, 941–951 (1987)
Berry, B. et al. Estrogen-responsive element of the human pS2 gene is an imperfectly palindromic sequence. Proc. Natl Acad. Sci USA 86, 1218–1222 (1989)
Migliaccio, A. et al. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 15, 1292–1300 (1996)
Catoria, G. et al. Non-transcriptional action of oestradiol and orogestrin triggers DNA synthesis. EMBO J. 18, 2500–2510 (1999)
Song, R.-X. et al. Linkage of estrogen action to MAP kinase activation through ERα association with Shc signalling pathway. Mol. Endocrinol. 16, 116–127 (2002)
Mandal, M. et al. Growth factors regulation of heterogeneous nuclear ribonucleoprotein expression and functions. J. Biol. Chem. 276, 9699–9704 (2001)
Acknowledgements
We thank B. S. Katzenellenbogen and M. R. Parker for AF2 fusion protein; D. McDonnell for 3X-ERE TATA; D. Shapiro for ERE-reporter systems; D. Nguyen for cloning; and W. Schmid for the GRE-CAT promoter constructs. Susan G. Komen Breast Cancer Foundation and National Institutes of Health grants (to R.K.) supported this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Kumar, R., Wang, RA., Mazumdar, A. et al. A naturally occurring MTA1 variant sequesters oestrogen receptor-α in the cytoplasm. Nature 418, 654–657 (2002). https://doi.org/10.1038/nature00889
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature00889
This article is cited by
-
Molecular characterization of Wdr13 knockout female mice uteri: a model for human endometrial hyperplasia
Scientific Reports (2020)
-
Cytoplasmic ERα and NFκB Promote Cell Survival in Mouse Mammary Cancer Cell Lines
Hormones and Cancer (2020)
-
Structure and function insights into the NuRD chromatin remodeling complex
Cellular and Molecular Life Sciences (2015)
-
Role of MTA1 in cancer progression and metastasis
Cancer and Metastasis Reviews (2014)
-
Metastatic tumor antigen in hepatocellular carcinoma: golden roads toward personalized medicine
Cancer and Metastasis Reviews (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.