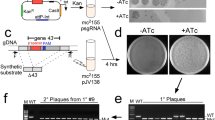
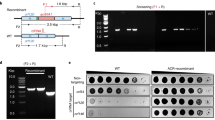
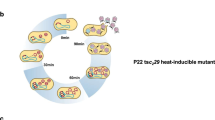
Abstract
Many bacteriophages and animal viruses integrate their genomes into the chromosomal DNA of their hosts as a method of promoting vertical transmission. Phages that integrate in a site-specific fashion encode an integrase enzyme that catalyses recombination between the phage and host genomes1,2. CTXφ is a filamentous bacteriophage that contains the genes encoding cholera toxin, the principal virulence factor of the diarrhoea-causing Gram-negative bacterium Vibrio cholerae3. CTXφ integrates into the V. cholerae genome in a site-specific manner4,5; however, the ∼6.9-kilobase (kb) CTXφ genome does not encode any protein with significant homology to known recombinases. Here we report that XerC and XerD, two chromosome-encoded recombinases that ordinarily function to resolve chromosome dimers at the dif recombination site6, are essential for CTXφ integration into the V. cholerae genome. The CTXφ integration site was found to overlap with the dif site of the larger of the two V. cholerae chromosomes. Examination of sequences of the integration sites of other filamentous phages indicates that the XerCD recombinases also mediate the integration of these phage genomes at dif-like sites in various bacterial species.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nash, H. A. in Escherichia coli and Salmonella (ed. Neidhardt, F. C.) 2363–2376 (ASM Press, Washington DC, 1996)
Campbell, A. M. Chromosomal insertion sites for phages and plasmids. J. Bacteriol. 174, 7495–7499 (1992)
Waldor, M. K. & Mekalanos, J. J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272, 1910–1914 (1996)
Pearson, G. D., Woods, A., Chiang, S. L. & Mekalanos, J. J. CTX genetic element encodes a site-specific recombination system and an intestinal colonization factor. Proc. Natl Acad. Sci. USA 90, 3750–3754 (1993)
Davis, B. M., Kimsey, H. H., Chang, W. & Waldor, M. K. The Vibrio cholerae O139 Calcutta bacteriophage CTXφ is infectious and encodes a novel repressor. J. Bacteriol. 181, 6779–6787 (1999)
Blakely, G. et al. Two related recombinases are required for site-specific recombination at dif and cer in E. coli K12. Cell 75, 351–361 (1993)
Heidelberg, J. F. et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406, 477–483 (2000)
Kuempel, P. L., Henson, J. M., Dircks, L., Tecklenburg, M. & Lim, D. F. dif, a recA-independent recombination site in the terminus region of the chromosome of Escherichia coli. New Biol. 3, 799–811 (1991)
Davis, B. M., Moyer, K. E., Boyd, E. F. & Waldor, M. K. CTX prophages in classical biotype Vibrio cholerae: functional phage genes but dysfunctional phage genomes. J. Bacteriol. 182, 6992–6998 (2000)
Tecklenburg, M., Naumer, A., Nagappan, O. & Kuempel, P. The dif resolvase locus of the Escherichia coli chromosome can be replaced by a 33-bp sequence, but function depends on location. Proc. Natl Acad. Sci. USA 92, 1352–1356 (1995)
Steiner, W., Liu, G., Donachie, W. D. & Kuempel, P. The cytoplasmic domain of FtsK protein is required for resolution of chromosome dimers. Mol. Microbiol. 31, 579–583 (1999)
Moyer, K. E., Kimsey, H. H. & Waldor, M. K. Evidence for a rolling-circle mechanism of phage DNA synthesis from both replicative and integrated forms of CTXφ. Mol. Microbiol. 41, 311–323 (2001)
Davis, B. M. & Waldor, M. K. CTXφ contains a hybrid genome derived from tandemly integrated elements. Proc. Natl Acad. Sci. USA 97, 8572–8577 (2000)
Parkhill, J. et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413, 523–527 (2001)
Lin, N. T., Chang, R. Y., Lee, S. J. & Tseng, Y. H. Plasmids carrying cloned fragments of RF DNA from the filamentous phage φLf can be integrated into the host chromosome via site-specific integration and homologous recombination. Mol. Genet. Genom. 266, 425–435 (2001)
Dai, H., Chow, T. Y., Liao, H. J., Chen, Z. Y. & Chiang, K. S. Nucleotide sequences involved in the neolysogenic insertion of filamentous phage Cf16-v1 into the Xanthomonas campestris pv. citri chromosome. Virology 167, 613–620 (1988)
Simpson, A. J. et al. The genome sequence of the plant pathogen Xylella fastidiosa. Nature 406, 151–157 (2000)
Dillard, J. P. & Seifert, H. S. A variable genetic island specific for Neisseria gonorrhaae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol. Microbiol. 41, 263–277 (2001)
Metcalf, W. W. et al. Conditionally replicative and conjugative plasmids carrying lacZ alpha for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35, 1–13 (1996)
Lessl, M. et al. Dissection of IncP conjugative plasmid transfer: definition of the transfer region Tra2 by mobilization of the Tra1 region in trans. J. Bacteriol. 174, 2493–2500 (1992)
Horton, R. M., Hunt, H. D., Ho, S. N., Pullen, J. K. & Pease, L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77, 61–68 (1989)
Butterton, J. R. et al. Heterologous antigen expression in Vibrio cholerae vector strains. Infect. Immun. 63, 2689–2696 (1995)
Acknowledgements
We thank L. Arciszewska, A. Camilli, A. Campbell, B. Davis, A. Kane, D. Raychaudhuri, M. Russel and A. Sonenshein for helpful suggestions, and E. Vimr for communication of unpublished observations. We are grateful to A. Kane and the New England Medical Center GRASP Center for the preparation of plates and media. We acknowledge the support of NIH, Howard Hughes Medical Institute and the Pew Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests
Rights and permissions
About this article
Cite this article
Huber, K., Waldor, M. Filamentous phage integration requires the host recombinases XerC and XerD. Nature 417, 656–659 (2002). https://doi.org/10.1038/nature00782
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature00782
This article is cited by
-
Cryo-EM structure of ssDNA bacteriophage ΦCjT23 provides insight into early virus evolution
Nature Communications (2022)
-
Putative plasmid prophages of Bacillus cereus sensu lato may hold the key to undiscovered phage diversity
Scientific Reports (2021)
-
High quality reference genomes for toxigenic and non-toxigenic Vibrio cholerae serogroup O139
Scientific Reports (2019)
-
Complete nucleotide sequence of a new filamentous phage, Xf109, which integrates its genome into the chromosomal DNA of Xanthomonas oryzae
Archives of Virology (2017)
-
Cold adaptation regulated by cryptic prophage excision in Shewanella oneidensis
The ISME Journal (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.