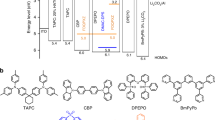
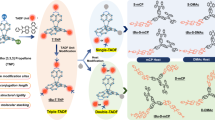
Abstract
Thermally activated delayed fluorescence (TADF) emitters, which produce light by harvesting both singlet and triplet excitons without noble metals, are emerging as next-generation organic electroluminescent materials. In the past few years, there have been rapid advances in molecular design criteria, our understanding of the photophysics underlying TADF and the applications of TADF materials as emitters in organic light-emitting diodes (OLEDs). This topic is set to remain at the forefront of research in optoelectronic organic materials for the foreseeable future. In this Review, we focus on state-of-the-art materials design and understanding of the photophysical processes, which are being leveraged to optimize the performance of OLED devices. Notably, we also appraise dendritic and polymeric TADF emitters — macromolecular materials that offer the potential advantages of low cost, solution processable and large-area OLED fabrication.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Tang, C. W. & VanSlyke, S. A. Organic electroluminescent diodes. Appl. Phys. Lett. 51, 913–915 (1987).This is a seminal paper describing the first OLEDs with a double-layer thin-film structure operating at a voltage below 10 V.
Friend, R. H. et al. Electroluminescence in conjugated polymers. Nature 397, 121–128 (1999).
Adachi, C., Baldo, M. A., Thompson, M. E. & Forrest, S. R. Nearly 100% internal phosphorescence efficiency in an organic light-emitting device. J. Appl. Phys. 90, 5048–5051 (2001).
Lee, C. W. & Lee, J. Y. Above 30% external quantum efficiency in blue phosphorescent organic light-emitting diodes using pyrido[2,3-b]indole derivatives as host materials. Adv. Mater. 25, 5450–5454 (2013).
Baldo, M. A. et al. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 395, 151–154 (1998).
Uoyama, H., Goushi, K., Shizu, K., Nomura, H. & Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238 (2012).This is a representative paper on TADF small molecules achieving nearly 100% IQE and nearly 20% electroluminescence efficiency.
Goushi, K., Yoshida, K., Sato, K. & Adachi, C. Organic light-emitting diodes employing efficient reverse intersystem crossing for triplet-to-singlet state conversion. Nat. Photon. 6, 253–258 (2012).
Zhang, Q. et al. Design of efficient thermally activated delayed fluorescence materials for pure blue organic light emitting diodes. J. Am. Chem. Soc. 134, 14706–14709 (2012).
Hedley, G. J., Ruseckas, A. & Samuel, I. D. W. Ultrafast luminescence in Ir(ppy)3 . Chem. Phys. Lett. 450, 292–296 (2008).
Lawetz, V., Siebrand, W. & Orlandi, G. J. Theory of intersystem crossing in aromatic hydrocarbons. Chem. Phys. 56, 4058–4072 (1972).
Robinson, G. W. & Frosch, R. P. Electronic excitation transfer and relaxation. J. Chem. Phys. 38, 1187–1203 (1963).
Kono, H., Lin, S. H. & Schlag, E. W. On the role of low-frequency modes in the energy or temperature dependence of intersystem crossing. Chem. Phys. Lett. 145, 280–285 (1988).
Fukumura, H., Kikuchi, K., Koike, K. & Kokubun, H. Temperature effect on inverse intersystem crossing of anthracenes. J. Photochem. Photobiol. A Chem. 42, 283–291 (1988).
Tanaka, F., Okamoto, M. & Hirayama, S. Pressure and temperature dependences of the rate constant for S1-T2 intersystem crossing of anthracene compounds in solution. J. Phys. Chem. 99, 525–530 (1995).
Ziegler, T., Rauk, A. & Baerends, E. J. On the calculation of multiplet energies by the Hartree-Fock-Slater method. Theor. Chim. Acta 43, 261–271 (1977).
El-Sayed, M. A. Spin-orbit coupling and the radiationless processes in nitrogen heterocyclics. J. Chem. Phys. 38, 2834–2838 (1963).
Czerwieniec, R., Leitl, M. J., Homeier, H. H. H. & Yersin, H. Cu(I) complexes — thermally activated delayed fluorescence. Photophysical approach and material design. Coord. Chem. Rev. 325, 2–28 (2016).
Pan, Y. et al. High yields of singlet excitons in organic electroluminescence through two paths of cold and hot excitons. Adv. Opt. Mater. 2, 510–515 (2014).
Wang, S. et al. Highly efficient near-infrared delayed fluorescence organic light emitting diodes using a phenanthrene-based charge-transfer compound. Angew. Chem. Int. Ed. 54, 13068–13072 (2015).
Samanta, P. K., Kim, D., Coropceanu, V. & Brédas, J.-L. Up-conversion intersystem crossing rates in organic emitters for thermally activated delayed fluorescence: impact of the nature of singlet versus triplet excited states. J. Am. Chem. Soc. 139, 4042–4051 (2017).
Dias, F. B. et al. Triplet harvesting with 100% efficiency by way of thermally activated delayed fluorescence in charge transfer OLED emitters. Adv. Mater. 25, 3707–3714 (2013).This study provides a systematic discussion of the factors affecting the efficiency of TADF small-molecule OLEDs, in which detailed molecular structure–photophysics relationships are revealed.
Malrieu, J.-P. & Pullman, B. Configuration spatiale et propriétés électroniques du noyau d’isoalloxazine. Theor. Chim. Acta 2, 302–314 (1964).
Aizenshtat, Z., Klein, E., Weiler-Feilchenfeld, H. & Bergmann, E. D. Conformational studies on xanthene, thioxanthene and acridan. Isr. J. Chem. 10, 753–763 (1972).
Zhang, Q. et al. Anthraquinone-based intramolecular charge-transfer compounds: computational molecular design, thermally activated delayed fluorescence, and highly efficient red electroluminescence. J. Am. Chem. Soc. 136, 18070–18081 (2014).
Endo, A. et al. Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes. Appl. Phys. Lett. 98, 083302 (2011).This study was the starting point for purely organic TADF emitters with a very small singlet–triplet gap.
Zhang, Q. et al. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photon. 8, 326–332 (2014).
Lee, D. R. et al. Design strategy for 25% external quantum efficiency in green and blue thermally activated delayed fluorescent devices. Adv. Mater. 27, 5861–5867 (2015).
Li, J. et al. Highly efficient organic light-emitting diode based on a hidden thermally activated delayed fluorescence channel in a heptazine derivative. Adv. Mater. 25, 3319–3323 (2013).
Lin, T. A. et al. Sky-blue organic light emitting diode with 37% external quantum efficiency using thermally activated delayed fluorescence from spiroacridine-triazine hybrid. Adv. Mater. 28, 6976–6983 (2016).This study demonstrates that the maximum EQE of TADF small-molecule OLEDs fabricated by vacuum deposition reaches 36.7%.
Tsai, W. L. et al. A versatile thermally activated delayed fluorescence emitter for both highly efficient doped and non-doped organic light emitting devices. Chem. Commun. 51, 13662–13665 (2015).
Im, Y. et al. Molecular design strategy of organic thermally activated delayed fluorescence emitters. Chem. Mater. 29, 1946–1963 (2017).
Hatakeyama, T. et al. Ultrapure blue thermally activated delayed fluorescence molecules: efficient HOMO-LUMO separation by the multiple resonance effect. Adv. Mater. 28, 2777–2781 (2016).
Cho, Y. J., Jeon, S. K., Lee, S.-S., Yu, E. & Lee, J. Y. Donor interlocked molecular design for fluorescence-like narrow emission in deep blue thermally activated delayed fluorescent emitters. Chem. Mater. 28, 5400–5405 (2016).
Lee, I. & Lee, J. Y. Molecular design of deep blue fluorescent emitters with 20% external quantum efficiency and narrow emission spectrum. Org. Electron. 29, 160–164 (2016).In this study, deep-blue TADF OLEDs are reported based on the rational design of the emitter molecule and host material.
Cui, L.-S. et al. Long-lived efficient delayed fluorescence organic light-emitting diodes using n-type hosts. Nat. Commun. 8, 2250 (2017).
Kim, H. M., Choi, J. M. & Lee, J. Y. Blue thermally activated delayed fluorescent emitters having a bicarbazole donor moiety. RSC Adv. 6, 64133–64139 (2016).
Lee, D. R., Choi, J. M., Lee, C. W. & Lee, J. Y. Ideal molecular design of blue thermally activated delayed fluorescent emitter for high efficiency, small singlet-triplet energy splitting, low efficiency roll-off, and long lifetime. ACS Appl. Mater. Interfaces 8, 23190–23196 (2016).
Parker, C. A. & Hatchard, C. G. Triplet-singlet emission in fluid solutions: phosphorescence of eosin. Trans. Faraday Soc. 57, 1894–1904 (1961).
Endo, A. et al. Thermally activated delayed fluorescence from Sn4+-porphyrin complexes and their application to organic light emitting diodes — a novel mechanism for electroluminescence. Adv. Mater. 21, 4802–4806 (2009).
Chen, X.-L. et al. Rational design of strongly blue-emitting cuprous complexes with thermally activated delayed fluorescence and application in solution-processed OLEDs. Chem. Mater. 25, 3910–3920 (2013).
Liang, D. et al. Highly efficient cuprous complexes with thermally activated delayed fluorescence for solution-processed organic light-emitting devices. Inorg. Chem. 55, 7467–7475 (2016).
Hofbeck, T. Monkowius, U. & Yersin, H. Highly efficient luminescence of Cu(I) compounds: thermally activated delayed fluorescence combined with short-lived phosphorescence. J. Am. Chem. Soc. 137, 399–404 (2015).
Volz, D. et al. Bridging the efficiency gap: fully bridged dinuclear Cu(I)-complexes for singlet harvesting in high-efficiency OLEDs. Adv. Mater. 27, 2538–2543 (2015).
Mehes, G., Nomura, H., Zhang, Q., Nakagawa, T. & Adachi, C. Enhanced electroluminescence efficiency in a spiro-acridine derivative through thermally activated delayed fluorescence. Angew. Chem. Int. Ed. 51, 11311–11315 (2012).
Kim, B. S. & Lee, J. Y. Engineering of mixed host for high external quantum efficiency above 25% in green thermally activated delayed fluorescence device. Adv. Funct. Mater. 24, 3970–3977 (2014).
Lee, D. R. et al. Above 30% external quantum efficiency in green delayed fluorescent organic light-emitting diodes. ACS Appl. Mater. Interfaces 7, 9625–9629 (2015).
Sun, J. W. et al. A fluorescent organic light-emitting diode with 30% external quantum efficiency. Adv. Mater. 26, 5684–5688 (2014).
Zeng, W. et al. Achieving nearly 30% external quantum efficiency for orange-red organic light emitting diodes by employing thermally activated delayed fluorescence emitters composed of 1, 8-naphthalimide-acridine hybrids. Adv. Mater. 30, 1704961 (2017).
Li, Y., Xie, G., Gong, S., Wu, K. & Yang, C. Dendronized delayed fluorescence emitters for non-doped, solution-processed organic light-emitting diodes with high efficiency and low efficiency roll-off simultaneously: two parallel emissive channels. Chem. Sci. 7, 5441–5447 (2016).
Ren, Z. et al. Pendant homopolymer and copolymers as solution-processable thermally activated delayed fluorescence materials for organic light-emitting diodes. Macromolecules 49, 5452–5460 (2016).In this study, the maximum EQE of a polymer OLED reaches 20% using the strategy of pendant TADF moieties in the side chain of a polymer.
Lee, S. Y., Yasuda, T., Yang, Y. S., Zhang, Q. & Adachi, C. Luminous butterflies: efficient exciton harvesting by benzophenone derivatives for full-color delayed fluorescence OLEDs. Angew. Chem. Inter. Ed. 53, 6402–6406 (2014).
Ban, X. et al. Design of encapsulated hosts and guests for highly efficient blue and green thermally activated delayed fluorescence OLEDs based on a solution-process. Chem. Commun. 53, 11834–11837 (2017).
Tsang, D. P. K., Matsushima, T. & Adachi, C. Operational stability enhancement in organic light-emitting diodes with ultrathin Liq interlayers. Sci. Rep. 6, 22463 (2016).
Cho, Y. J., Yook, K. S. & Lee, J. Y. A universal host material for high external quantum efficiency close to 25% and long lifetime in green fluorescent and phosphorescent OLEDs. Adv. Mater. 26, 4050–4055 (2014).
Wong, M. Y. & Zysman-Colman, E. Purely organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Adv. Mater. 29, 1605444 (2017).
Ritchie, J., Crayston, J. A., Markham, J. P. & Samuel, I. D. Effect of meta-linkages on the photoluminescence and electroluminescence properties of light-emitting polyfluorene alternating copolymers. J. Mater. Chem. 16, 1651–1656 (2006).
Ahn, T., Jang, M. S., Shim, H. K., Hwang, D. H. & Zyung, T. Blue electroluminescent polymers: control of conjugation length by kink linkages and substituents in the poly (p-phenylenevinylene)-related copolymers. Macromolecules 32, 3279–3285 (1999).
Tanaka, H., Shizu, K., Miyazaki, H. & Adachi, C. Efficient green thermally activated delayed fluorescence (TADF) from a phenoxazine-triphenyltriazine (PXZ-TRZ) derivative. Chem. Commun. 48, 11392–11394 (2012).
Kaji, H. et al. Purely organic electroluminescent material realizing 100% conversion from electricity to light. Nat. Commun. 6, 8476 (2015).
Shizu, K. et al. Highly efficient blue electroluminescence using delayed-fluorescence emitters with large overlap density between luminescent and ground states. J. Phys. Chem. C 119, 26283–26289 (2015).
Serevicius, T. et al. Enhanced electroluminescence based on thermally activated delayed fluorescence from a carbazole-triazine derivative. Phys. Chem. Chem. Phys. 15, 15850–15855 (2013).
Hirata, S. et al. Highly efficient blue electroluminescence based on thermally activated delayed fluorescence. Nat. Mater. 14, 330–336 (2015).This report reveals the compatibility between low ΔEst and high PLQY and produces a blue emitter with near 100% internal electroluminescence quantum yield.
Park, I. S., Lee, S. Y., Adachi, C. & Yasuda, T. Full-color delayed fluorescence materials based on wedge-shaped phthalonitriles and dicyanopyrazines: systematic design, tunable photophysical properties, and OLED performance. Adv. Funct. Mater. 26, 1813–1821 (2016).
Liu, M. et al. Blue thermally activated delayed fluorescence materials based on bis(phenylsulfonyl)benzene derivatives. Chem. Commun. 51, 16353–16356 (2015).
Wang, Z. et al. Structure-performance investigation of thioxanthone derivatives for developing color tunable highly efficient thermally activated delayed fluorescence emitters. ACS Appl. Mater. Interfaces 8, 8627–8636 (2016).
Kawasumi, K. et al. Thermally activated delayed fluorescence materials based on homoconjugation effect of donor-acceptor triptycenes. J. Am. Chem. Soc. 137, 11908–11911 (2015).
Nakagawa, T., Ku, S. Y., Wong, K. T. & Adachi, C. Electroluminescence based on thermally activated delayed fluorescence generated by a spirobifluorene donor-acceptor structure. Chem. Commun. 48, 9580–9582 (2012).
Chen, X.-K. et al. A new design strategy for efficient thermally activated delayed fluorescence organic emitters: from twisted to planar structures. Adv. Mater. 29, 1702767 (2017).
Nishimoto, T., Yasuda, T., Lee, S. Y., Kondo, R. & Adachi, C. A six-carbazole-decorated cyclophosphazene as a host with high triplet energy to realize efficient delayed-fluorescence OLEDs. Mater. Horiz. 1, 264–269 (2014).
Lin, M.-S. et al. Incorporation of a CN group into mCP: a new bipolar host material for highly efficient blue and white electrophosphorescent devices. J. Mater. Chem. 22, 16114–16120 (2012).
Cho, Y. J., Yook, K. S. & Lee, J. Y. High efficiency in a solution-processed thermally activated delayed-fluorescence device using a delayed-fluorescence emitting material with improved solubility. Adv. Mater. 26, 6642–6646 (2014).This paper pioneers solution-processed TADF small-molecule emitting materials: the corresponding OLED device exhibits a high electroluminescence efficiency of 18.3%.
Tsai, Y. S., Hong, L. A., Juang, F. S. & Chen, C. Y. Blue and white phosphorescent organic light emitting diode performance improvement by confining electrons and holes inside double emitting layers. J. Lumin. 153, 312–316 (2014).
Senes, A. et al. Increasing the horizontal orientation of transition dipole moments in solution processed small molecular emitters. J. Mater. Chem. C 5, 6555–6562 (2017).
Kim, B. S. & Lee, J. Y. Phosphine oxide type bipolar host material for high quantum efficiency in thermally activated delayed fluorescent device. ACS Appl. Mater. Interfaces 6, 8396–8400 (2014).
Seino, Y., Inomata, S., Sasabe, H., Pu, Y. J. & Kido, J. High-performance green OLEDs using thermally activated delayed fluorescence with a power efficiency of over 100 lm W. Adv. Mater. 28, 2638–2643 (2016).
Gaj, M. P., Fuentes-Hernandez, C., Zhang, Y., Marder, S. R. & Kippelen, B. Highly efficient organic light-emitting diodes from thermally activated delayed fluorescence using a sulfone–carbazole host material. Org. Electron. 16, 109–112 (2015).
Fan, C. et al. Dibenzothiophene-based phosphine oxide host and electron-transporting materials for efficient blue thermally activated delayed fluorescence diodes through compatibility optimization. Chem. Mater. 27, 5131–5140 (2015).
Ding, D., Zhang, Z., Wei, Y., Yan, P. & Xu, H. Spatially optimized quaternary phosphine oxide host materials for high-efficiency blue phosphorescence and thermally activated delayed fluorescence organic light-emitting diodes. J. Mater. Chem. C 3, 11385–11396 (2015).
Zhang, J. et al. Multiphosphine-oxide hosts for ultralow-voltage-driven true-blue thermally activated delayed fluorescence diodes with external quantum efficiency beyond 20%. Adv. Mater. 28, 479–485 (2016).
Dos Santos, P. et al. Using guest-host interactions to optimize the efficiency of TADF OLEDs. J. Phys. Chem. Lett. 7, 3341–3346 (2016).
Nishide, J., Nakanotani, H., Hiraga, Y. & Adachi, C. High-efficiency white organic light-emitting diodes using thermally activated delayed fluorescence. Appl. Phys. Lett. 104, 233304 (2014).
Zhang, D., Duan, L., Li, Y., Zhang, D. & Qiu, Y. Highly efficient and color-stable hybrid warm white organic light-emitting diodes using a blue material with thermally activated delayed fluorescence. J. Mater. Chem. C 2, 8191–8197 (2014).
Li, X. L. et al. High-efficiency WOLEDs with high color-rendering index based on a chromaticity-adjustable yellow thermally activated delayed fluorescence emitter. Adv. Mater. 28, 4614–4619 (2016).
Kim, S.-Y. et al. Organic light-emitting diodes with 30% external quantum efficiency based on a horizontally oriented emitter. Adv. Funct. Mater. 23, 3896–3900 (2013).
Nowy, S., Krummacher, B. C., Frischeisen, J., Reinke, N. A. & Brütting, W. Light extraction and optical loss mechanisms in organic light-emitting diodes: Influence of the emitter quantum efficiency. J. Appl. Phys. 104, 123109 (2008).
Mayr, C. et al. Efficiency enhancement of organic light-emitting diodes incorporating a highly oriented thermally activated delayed fluorescence emitter. Adv. Funct. Mater. 24, 5232–5239 (2014).
Zhang, Q. et al. Nearly 100% internal quantum efficiency in undoped electroluminescent devices employing pure organic emitters. Adv. Mater. 27, 2096–2100 (2015).
Guo, J. et al. Achieving high-performance nondoped OLEDs with extremely small efficiency roll-off by combining aggregation-induced emission and thermally activated delayed fluorescence. Adv. Funct. Mater. 27, 1606458 (2017).
Huang, J. et al. Highly efficient nondoped OLEDs with negligible efficiency roll-off fabricated from aggregation-induced delayed fluorescence luminogens. Angew. Chem. Int. Ed. 129, 13151–13156 (2017).
Goushi, K. & Adachi, C. Efficient organic light-emitting diodes through up-conversion from triplet to singlet excited states of exciplexes. Appl. Phys. Lett. 101, 023306 (2012).
Liu, X. K. et al. Prediction and design of efficient exciplex emitters for high-efficiency, thermally activated delayed-fluorescence organic light-emitting diodes. Adv. Mater. 27, 2378–2383 (2015).
Liu, W. et al. Novel strategy to develop exciplex emitters for high-performance OLEDs by employing thermally activated delayed fluorescence materials. Adv. Funct. Mater. 26, 2002–2008 (2016).
Duan, L. et al. Solution processable small molecules for organic light-emitting diodes. J. Mater. Chem. 20, 6392–6407 (2010).
Cho, Y. J., Chin, B. D., Jeon, S. K. & Lee, J. Y. 20% external quantum efficiency in solution-processed blue thermally activated delayed fluorescent devices. Adv. Funct. Mater. 25, 6786–6792 (2015).
Mei, L. et al. The inductive-effect of electron withdrawing trifluoromethyl for thermally activated delayed fluorescence: tunable emission from tetra- to penta-carbazole in solution processed blue OLEDs. Chem. Commun. 51, 13024–13027 (2015).
Chen, P. et al. Delayed fluorescence in a solution-processable pure red molecular organic emitter based on dithienylbenzothiadiazole: a joint optical, electroluminescence, and magneto electroluminescence study. ACS Appl. Mater. Interfaces 7, 2972–2978 (2015).
Wada, Y. et al. Highly efficient electroluminescence from a solution-processable thermally activated delayed fluorescence emitter. Appl. Phys. Lett. 107, 183303 (2015).
Xie, G. et al. Evaporation- and solution-process-feasible highly efficient thianthrene-9,9′,10,10′-tetraoxide-based thermally activated delayed fluorescence emitters with reduced efficiency roll-off. Adv. Mater. 28, 181–187 (2016).
Schmidbauer, S., Hohenleutner, A. & König, B. Chemical degradation in organic light-emitting devices: mechanisms and implications for the design of new materials. Adv. Mater. 25, 2114–2129 (2013).
Cui, L. S. et al. Pure hydrocarbon hosts for ≈ 100% exciton harvesting in both phosphorescent and fluorescent light-emitting devices. Adv. Mater. 27, 4213–4217 (2015).
Cui, L. S. et al. Controlling synergistic oxidation processes for efficient and stable blue thermally activated delayed fluorescence devices. Adv. Mater. 28, 7620–7625 (2016).
Kanibolotsky, A. L., Perepichka, I. F. & Skabara, P. J. Star-shaped π-conjugated oligomers and their applications in organic electronics and photonics. Chem. Soc. Rev. 39, 2695–2728 (2010).
Burn, P. L., Lo, S. C. & Samuel, D. W. The development of light-emitting dendrimers for displays. Adv. Mater. 19, 1675–1688 (2007).
Albrecht, K., Matsuoka, K., Fujita, K. & Yamamoto, K. Carbazole dendrimers as solution-processable thermally activated delayed-fluorescence materials. Angew. Chem. Int. Ed. 54, 5677–5682 (2015).In this study, a solution-processed dendrimer TADF emitter is reported with a maximum EQE of 3.4%.
Luo, J. et al. Multi-carbazole encapsulation as a simple strategy for the construction of solution-processed, non-doped thermally activated delayed fluorescence emitters. J. Mater. Chem. C 4, 2442–2446 (2016).
Albrecht, K. et al. Thermally activated delayed fluorescence OLEDs with fully solution processed organic layers exhibiting nearly 10% external quantum efficiency. Chem. Commun. 53, 2439–2442 (2017).
Li, J. et al. Deep-blue thermally activated delayed fluorescence dendrimers with reduced singlet-trip let energy gap for low roll-off non-doped solution-processed organic light-emitting diodes. Dyes Pigments 140, 79–86 (2017).
Matsuoka, K., Albrecht, K., Yamamoto, K. & Fujita, K. Mulifunctional dendritic emitter: aggregation-induced emission enhanced, thermally activated delayed fluorescent material for solution-processed multilayered organic light-emitting diodes. Sci. Rep. 7, 41780 (2017).
Ban, X. et al. Self-host thermally activated delayed fluorescent dendrimers with flexible chains: an effective strategy for non-doped electroluminescent devices based on solution processing. J. Mater. Chem. C 4, 8810–8816 (2016).
Ban, X., Jiang, W., Sun, K., Lin, B. & Sun, Y. Self-host blue dendrimer comprised of thermally activated delayed fluorescence core and bipolar dendrons for efficient solution-processable nondoped electroluminescence. ACS Appl. Mater. Interfaces 9, 7339–7346 (2017).
Sun, K. et al. Design strategy of yellow thermally activated delayed fluorescent dendrimers and their highly efficient non-doped solution-processed OLEDs with low driving voltage. Org. Electron. 42, 123–130 (2017).
Godumala, M. et al. Novel dendritic large molecules as solution-processable thermally activated delayed fluorescent emitters for simple structured non-doped organic light emitting diodes. J. Mater. Chem. C 6, 1160–1170 (2018).
Sun, K. et al. Thermally activated delayed fluorescence dendrimers with exciplex-forming dendrons for low-voltage-driving and power-efficient solution-processed OLEDs. J. Mater. Chem. C 6, 43–49 (2018).
Wang, Y. et al. Engineering the interconnecting position of star-shaped donor-pi-acceptor molecules based on triazine, spirofluorene, and triphenylamine moieties for color tuning from deep blue to green. Chem. Asian J. 11, 2555–2563 (2016).
Xia, D. et al. Self-host blue-emitting Iridium dendrimer with carbazole dendrons: nondoped phosphorescent organic light-emitting diodes. Angew. Chem. Int. Ed. 53, 1048–1052 (2014).
Chen, L. et al. Self-host heteroleptic green iridium dendrimers: achieving efficient non-doped device performance based on a simple molecular structure. Chem. Commun. 47, 9519–9521 (2011).
Albrecht, K., Kasai, Y., Kimoto, A. & Yamamoto, K. The synthesis and properties of carbazole-phenylazomethine double layer-type dendrimers. Macromolecules 41, 3793–3800 (2008).
Zhao, Z. H. et al. Synthesis and properties of dendritic emitters with a fluorinated starburst oxadiazole core and twisted carbazole dendrons. Macromolecules 44, 1405–1413 (2011).
Ding, J. et al. Bifunctional green Iridium dendrimers with a “self-host” feature for highly efficient nondoped electrophosphorescent devices. Angew. Chem. Int. Ed. 48, 6664–6666 (2009).
Albrecht, K., Pernites, R., Felipe, M. J., Advincula, R. C. & Yamamoto, K. Patterning carbazole-phenylazomethine dendrimer films. Macromolecules 45, 1288–1295 (2012).
Kimoto, A. et al. Novel hole-transport material for efficient polymer light-emitting diodes by photoreaction. Macromol. Rapid Commun. 26, 597–601 (2005).
Yook, K. S. & Lee, J. Y. Small molecule host materials for solution processed phosphorescent organic light-emitting diodes. Adv. Mater. 26, 4218–4233 (2014).
Chen, D. et al. Fluorescent organic planar p-n heterojunction light-emitting diodes with simplified structure, extremely low driving voltage, and high efficiency. Adv. Mater. 28, 239–244 (2016).
Sekine, C., Tsubata, Y., Yamada, T., Kitano, M. & Doi, S. Recent progress of high performance polymer OLED and OPV materials for organic printed electronics. Sci. Technol. Adv. Mater. 15, 34203 (2014).
Grimsdale, A. C., Chan, K. L., Martin, R. E., Jokisz, P. G. & Holmes, A. B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 109, 897–1091 (2009).
Rothe, C. & Monkman, A. Regarding the origin of the delayed fluorescence of conjugated polymers. J. Chem. Phys. 123, 244904 (2005).
Lee, S. Y., Yasuda, T., Komiyama, H., Lee, J. & Adachi, C. Thermally activated delayed fluorescence polymers for efficient solution-processed organic light-emitting diodes. Adv. Mater. 28, 4019–4024 (2016).
Zhu, Y. et al. Synthesis and electroluminescence of a conjugated polymer with thermally activated delayed fluorescence. Macromolecules 49, 4373–4377 (2016).
Nikolaenko, A. E., Cass, M., Bourcet, F., Mohamad, D. & Roberts, M. Thermally activated delayed fluorescence in polymers: a new route toward highly efficient solution processable OLEDs. Adv. Mater. 27, 7236–7240 (2015).
Nobuyasu, R. S. et al. Rational design of TADF polymers using a donor-acceptor monomer with enhanced TADF efficiency induced by the energy alignment of charge transfer and local triplet excited states. Adv. Opt. Mater. 4, 597–607 (2016).
Wang, Y. et al. Bright white electroluminescence from a single polymer containing a thermally activated delayed fluorescence unit and a solution-processed orange OLED approaching 20% external quantum efficiency. J. Mater. Chem. C 5, 10715–10720 (2017).
Wang, Y. et al. Efficient non-doped yellow OLEDs based on thermally activated delayed fluorescence conjugated polymers with an acridine/carbazole donor backbone and triphenyltriazine acceptor pendant. J. Mater. Chem. C 6, 568–574 (2018).
Luo, J., Xie, G., Gong, S., Chen, T. & Yang, C. Creating a thermally activated delayed fluorescence channel in a single polymer system to enhance exciton utilization efficiency for bluish-green electroluminescence. Chem. Commun. 52, 2292–2295 (2016).
Xie, G. et al. Inheriting the characteristics of TADF small molecule by side-chain engineering strategy to enable bluish-green polymers with high PLQYs up to 74% and external quantum efficiency over 16% in light-emitting diodes. Adv. Mater. 29, 1604223 (2017).
Sun, K. et al. Thermally cross-linkable thermally activated delayed fluorescent materials for efficient blue solution-processed organic light-emitting diodes. J. Mater. Chem. C 4, 8973–8979 (2016).
Shao, S. et al. Blue thermally activated delayed fluorescence polymers with nonconjugated backbone and through-space charge transfer effect. J. Am. Chem. Soc. 139, 17739–17742 (2017).
Köhler, A. & Beljonne, D. The singlet-triplet exchange energy in conjugated polymers. Adv. Funct. Mater. 14, 11–18 (2004).
Wei, Q. et al. Conjugation-induced thermally activated delayed fluorescence (TADF): from conventional non-TADF units to TADF-active polymers. Adv. Funct. Mater. 27, 1605051 (2017).
Reineke, S. Organic light-emitting diodes: Phosphorescence meets its match. Nat. Photon. 8, 269–270 (2014).
Dias, F. B. Kinetics of thermal-assisted delayed fluorescence in blue organic emitters with large singlet-triplet energy gap. Phil. Trans. R. Soc. A 373, 20140447 (2015).
Uchida, M., Adachi, M., Koyama, T. & Taniguchi, Y. Charge carrier trapping effect by luminescent dopant molecules in single-layer organic light emitting diodes. J. Appl. Phys. 86, 1680–1687 (1999).
Tsung, K. K. & So, S. K. Carrier trapping and scattering in amorphous organic hole transporter. Appl. Phys. Lett. 92, 103315 (2008).
Wang, S. et al. Ultrahigh color-stable, solution-processed, white OLEDs using a dendritic binary host and long-wavelength dopants with different charge trapping depths. Adv. Opt. Mater. 3, 1349–1354 (2015).
Zhang, Y. & Forrest, S. R. Triplets contribute to both an increase and loss in fluorescent yield in organic light emitting diodes. Phys. Rev. Lett. 108, 267404 (2012).
Lin, X. et al. Highly efficient TADF polymer electroluminescence with reduced efficiency roll-off via interfacial exciplex host strategy. ACS Appl. Mater. Interfaces 10, 47–52 (2018).
Murawski, C., Leo, K. & Gather, M. C. Efficiency roll-off in organic light-emitting diodes. Adv. Mater. 25, 6801–6827 (2013).
Fu, Q., Chen, J., Shi, C. & Ma, D. Solution-processed small molecules as mixed host for highly efficient blue and white phosphorescent organic light-emitting diodes. ACS Appl. Mater. Interfaces 4, 6579–6586 (2012).
Wu, W., Tang, R., Li, Q. & Li, Z. Functional hyperbranched polymers with advanced optical, electrical and magnetic properties. Chem. Soc. Rev. 44, 3997–4022 (2015).
Li, C. et al. Thermally activated delayed fluorescence pendant copolymers with electron and hole-transporting spacers. ACS Appl. Mater. Interfaces 10, 5731–5739 (2018).
Ward, J. S. et al. The interplay of thermally activated delayed fluorescence (TADF) and room temperature organic phosphorescence in sterically-constrained donor-acceptor charge-transfer molecules. Chem. Commun. 52, 2612–2615 (2016).
Furue, R., Nishimoto, T., Park, I. S., Lee, J. & Yasuda, T. Aggregation-induced delayed fluorescence based on donor/acceptor-tethered janus carborane triads: unique photophysical properties of nondoped OLEDs. Angew. Chem. Int. Ed. 128, 7287–7291 (2016).
Li, C. et al. Solution-processable thermally activated delayed fluorescence white OLEDs based on dual-emission polymers with tunable emission colors and aggregation-enhanced emission properties. Adv. Opt. Mater. 5, 1700435 (2017).This report provides a novel route of combining TADF and aggregation-induced emission in a polymeric emitter to enhance the efficiency.
Shinar, J. & Shinar, R. Organic light-emitting devices (OLEDs) and OLED-based chemical and biological sensors: an overview. J. Phys. D Appl. Phys. 41, 133001 (2008).
Freeman, D. M. E. et al. Synthesis and exciton dynamics of donor-orthogonal acceptor conjugated polymers: reducing the singlet-triplet energy gap. J. Am. Chem. Soc. 139, 11073–11080 (2017).
Xie, Y. & Li, Z. Thermally activated delayed fluorescent polymers. J. Polym. Sci. Part A Polym. Chem. 55, 575–584 (2017).
Huang, S. et al. Computational prediction for singlet- and triplet-transition energies of charge-transfer compounds. J. Chem. Theory Comput. 9, 3872–3877 (2013).
Peng, Q. et al. Theoretical study of conversion and decay processes of excited triplet and singlet states in a thermally activated delayed fluorescence molecule. J. Phys. Chem. C 121, 13448–13456 (2017).
Tian, X., Sun, H., Zhang, Q. & Adachi, C. Theoretical predication for transition energies of thermally activated delayed fluorescence molecules. Chinese Chem. Lett. 27, 1445–1452 (2016).
Chen, X. K., Zhang, S. F., Fan, J. X. & Ren, A. M. Nature of highly efficient thermally activated delayed fluorescence in organic light-emitting diode emitters: nonadiabatic effect between excited states. J. Phys. Chem. C 119, 9728–9733 (2015).
Marian, C. M. Mechanism of the triplet-to-singlet up conversion in the assistant dopant Acrxtn. J. Phys. Chem. C 120, 3715–3721 (2016).
Nakanotani, H. et al. High-efficiency organic light-emitting diodes with fluorescent emitters. Nat. Commun. 5, 4016 (2014).
Meng, L. et al. Highly efficient nondoped organic light emitting diodes based on thermally activated delayed fluorescence emitter with quantum well structure. ACS Appl. Mater. Interfaces 8, 20955–20961 (2016).
Acknowledgements
The financial support of the National Natural Science Foundations of China under Grant Nos. 51521062 and 21274009 (Z.R. and S.Y.) and the Engineering and Physical Sciences Research Council (EPSRC) Grant No. EL/L02621X/1 (M.R.B.) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and contributed to the discussion of content, as well as the writing and editing of the article, before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Supplementary information
Supplementary Figures (PDF 522 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Li, C., Ren, Z. et al. All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Nat Rev Mater 3, 18020 (2018). https://doi.org/10.1038/natrevmats.2018.20
Published:
DOI: https://doi.org/10.1038/natrevmats.2018.20
This article is cited by
-
Efficient, narrow-band, and stable electroluminescence from organoboron-nitrogen-carbonyl emitter
Nature Communications (2024)
-
A database of thermally activated delayed fluorescent molecules auto-generated from scientific literature with ChemDataExtractor
Scientific Data (2024)
-
Fluorene-based conjugates with geminal donor-acceptor: synthesis, photophysical properties and theoretical studies
Journal of Chemical Sciences (2024)
-
Recent Progress in Phenoxazine-Based Thermally Activated Delayed Fluorescent Compounds and Their Full-Color Organic Light-Emitting Diodes
Topics in Current Chemistry (2024)
-
High-efficiency and stable short-delayed fluorescence emitters with hybrid long- and short-range charge-transfer excitations
Nature Communications (2023)