Abstract
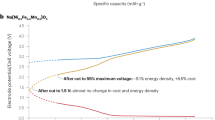
Sodium-ion batteries have been identified as appealing alternatives to lithium-ion batteries because they are made from raw materials that are less expensive, more abundant and less toxic. However, the frequently discussed cost advantage of sodium-ion batteries has, so far, not been examined in detail. In this Perspective, we use the Battery Performance and Cost (BatPaC) model to undertake a cost analysis of the materials for sodium-ion and lithium-ion cells, as well as complete batteries, and determine the effect of exchanging lithium with sodium, as well as the effect of replacing the material used for the anode current collector foil, on the cost. Moreover, we compare the calculated production costs of exemplary sodium-ion and lithium-ion batteries and highlight the most relevant parameters for optimization. Finally, the major raw materials for lithium-ion cathodes are examined in terms of potential supply risks because supply issues may lead to increased costs. Through the use of a scenario-based supply and demand analysis, the risks to the supply of lithium and cobalt are assessed, and implications for battery research are discussed. Overall, we provide a broad and interdisciplinary perspective on modern batteries and future directions for this field, with a focus on sodium-ion batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Armand, M. & Tarascon, J.M. Building better batteries. Nature 451, 652–657 (2008).
Yoshino, A. The birth of the lithium-ion battery. Angew. Chemie Int. Ed. 51, 5798–5800 (2012).
Scrosati, B. History of lithium batteries. J. Solid State Electrochem. 15, 1623–1630 (2011).
Nishi, Y. The development of lithium ion secondary batteries. Chem. Rec. 1, 406–413 (2001).
Nishi, Y. in Lithium-Ion Batteries (eds Yoshio, M., Brodd, R. J. & Kozawa, A. ) v–vii (Springer, New York, 2009).
Blomgren, G. E. The development and future of lithium ion batteries. J. Electrochem. Soc. 164, A5019–A5025 (2017).
Murray, J. L. The Al−Na (aluminum−sodium) system. Bull. Alloy Phase Diagrams 4, 407–410 (1983).
Larcher, D. & Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 7, 19–29 (2014).
United States Geological Survey. Mineral commodity summaries 2018. USGS Mineral Resources Program https://minerals.usgs.gov/minerals/pubs/mcs/2018/mcs2018.pdf (2018).
Gruber, P. W. et al. Global lithium availability. J. Ind. Ecol. 15, 760–775 (2011).
Yabuuchi, N., Kubota, K., Dahbi, M. & Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 114, 11636–11682 (2014).
Kim, Y., Ha, K.H., Oh, S. M. & Lee, K. T. High-capacity anode materials for sodium-ion batteries. Chem. Eur. J. 20, 11980–11992 (2014).
Berg, E. J., Villevieille, C., Streich, D., Trabesinger, S. & Novák, P. Rechargeable batteries: grasping for the limits of chemistry. J. Electrochem. Soc. 162, A2468–A2475 (2015).
Choi, J. W. & Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 1, 16013 (2016).
Nelson, P. A., Gallagher, K. G., Bloom, I. & Dees, D. W. Modeling the performance and cost of lithium-ion batteries for electric-drive vehicles. Argonne National Laboratory http://www.ipd.anl.gov/anlpubs/2011/10/71302.pdf (2012).
Gaines, L. & Cuenca, R. Costs of lithium-ion batteries for vehicles. Argonne National Laboratory https://anl.app.box.com/s/6ae31n7z9mop9opwp477mqpkcykljysr (2000).
Ciez, R. E. & Whitacre, J. F. The cost of lithium is unlikely to upend the price of Li-ion storage systems. J. Power Sources 320, 310–313 (2016).
United States Geological Survey. Mineral Commodity Summaries 2017. USGS Mineral Resources Program https://minerals.usgs.gov/minerals/pubs/mcs/2017/mcs2017.pdf (2017).
United States Geological Survey. Metal Prices in the United States Through 2010: U. S. Geological Survey Scientific Investigations Report 2012–5188. USGS Publications Warehouse https://pubs.usgs.gov/sir/2012/5188/sir2012-5188.pdf (2013).
Billaud, J. et al. β-NaMnO2: a high-performance cathode for sodium-ion batteries. J. Am. Chem. Soc. 136, 17243–17248 (2014).
Kim, Y. et al. An amorphous red phosphorus/carbon composite as a promising anode material for sodium ion batteries. Adv. Mater. 25, 3045–3049 (2013).
Li, W. et al. Confined amorphous red phosphorus in MOF-derived N-doped microporous carbon as a superior anode for sodium-ion battery. Adv. Mater. 29, 1605820 (2017).
Baumann, M., Peters, J. F., Weil, M. & Grunwald, A. CO2 footprint and life-cycle costs of electrochemical energy storage for stationary grid applications. Energy Technol. 5, 1071–1083 (2017).
Peters, J. F., Baumann, M., Zimmermann, B., Braun, J. & Weil, M. The environmental impact of Li-ion batteries and the role of key parameters — a review. Renew. Sustain. Energy Rev. 67, 491–506 (2017).
Mu, L. et al. Prototype sodium-ion batteries using an air-stable and Co/Ni-free O3 layered metal oxide cathode. Adv. Mater. 27, 6928–6933 (2015).
The National Center for Scientific Research. A promising new prototype of battery. CNRS http://www2.cnrs.fr/en/2659.htm (2015).
Keller, M., Buchholz, D. & Passerini, S. Layered Na-ion cathodes with outstanding performance resulting from the synergetic effect of mixed P- and O-type phases. Adv. Energy Mater. 6, 1501555 (2016).
Keller, M., Vaalma, C., Buchholz, D. & Passerini, S. Development and characterization of high-performance sodium-ion cells based on layered oxide and hard carbon. ChemElectroChem 3, 1124–1132 (2016).
Xia, X. & Dahn, J. R. NaCrO2 is a fundamentally safe positive electrode material for sodium-ion batteries with liquid electrolytes. Electrochem. Solid-State Lett. 15, A1–A4 (2012).
Kondou, H., Kim, J. & Watanabe, H. Thermal analysis on Na plating in sodium ion battery. Electrochemistry 85, 647–649 (2017).
Arora, P., White, R. E. & Doyle, M. Capacity fade mechanisms and side reactions in lithium-ion batteries. J. Electrochem. Soc. 145, 3647–3667 (1998).
Guo, R., Lu, L., Ouyang, M. & Feng, X. Mechanism of the entire overdischarge process and overdischarge-induced internal short circuit in lithium-ion batteries. Sci. Rep. 6, 30248 (2016).
Komaba, S., Hasegawa, T., Dahbi, M. & Kubota, K. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors. Electrochem. Commun. 60, 172–175 (2015).
Eshetu, G. G. et al. Comprehensive insights into the reactivity of electrolytes based on sodium ions. ChemSusChem 9, 462–471 (2016).
Paulsen, J., Komaba, S., Hara, R., Yabuuchi, N. & Kajiyama, M. EP2962346 (2014).
Kesler, S. E. et al. Global lithium resources: relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 48, 55–69 (2012).
Nassar, N. T., Graedel, T. E. & Harper, E. M. Byproduct metals are technologically essential but have problematic supply. Sci. Adv. 1, e1400180 (2015).
Evans, K. in Critical Metals Handbook (ed. Gunn, G. ) 230–260 (Wiley, Oxford, 2014).
An, J. W. et al. Recovery of lithium from Uyuni salar brine. Hydrometallurgy 117–118, 64–70 (2012).
Vikström, H., Davidsson, S. & Höö k, M. Lithium availability and future production outlooks. Appl. Energy 110, 252–266 (2013).
Galaxy Resources. Annual Report 2012. Galaxy Resources http://www.galaxyresources.com.au/Investor/gxy_ar_dec2012.pdf (2013).
The Cobalt Institute. Application of cobalt in rechargeable batteries. The Cobalt Institute https://www.cobaltinstitute.org/rechargeable-batteries.html (2018).
Weil, M. & Ziemann, S. in Lithium-Ion Batteries (ed. Pistoia, G. ) 509–528 (Elsevier, Amsterdam, 2014).
Amnesty International. ‘This is what we die for.’ Human rights abuses in the Democratic Republic of the Congo power the global trade in cobalt. Amnesty International https://www.amnestyusa.org/files/this_what_we_die_for_-_report.pdf (2016).
Pillot, C. Lithium ion battery raw material supply & demand 2016–2025. Avicenne Energy http://cii-resource.com/cet/AABE-03-17/Presentations/BRMT/Pillot_Christophe.pdf (2017).
Roland Berger Strategy Consultants. The lithium-ion battery value chain. Peter Sauber Argentur http://www.messe-sauber.de/doc/files/e4_1_kruger_script.pdf (2012).
Chung, D., Elgqvist, E. & Santhanagopalan, S. Automotive lithium-ion cell manufacturing: regional cost structures and supply chain considerations. National Renewable Energy Laboratory https://www.nrel.gov/docs/fy16osti/66086.pdf (2016).
Acknowledgements
The authors thank L. Grande for fruitful discussions and acknowledge the financial support of the Helmholtz Association. J. Riegert and K. Peters are acknowledged for their help with preparing the manuscript text and figures before submission.
Author information
Authors and Affiliations
Contributions
C.V. researched the data and conducted the calculations. C.V. and D.B. wrote the manuscript. All authors contributed to the discussion of the results and implications and commented on the manuscript at all stages. S.P. edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Supplementary information
Supplementary Methods Figures and Tables (PDF 3820 kb)
Supplementary information
Supplementary Dataset (XLSX 54 kb)
Rights and permissions
About this article
Cite this article
Vaalma, C., Buchholz, D., Weil, M. et al. A cost and resource analysis of sodium-ion batteries. Nat Rev Mater 3, 18013 (2018). https://doi.org/10.1038/natrevmats.2018.13
Published:
DOI: https://doi.org/10.1038/natrevmats.2018.13
This article is cited by
-
Exploration of the two-dimensional transition metal phosphide MoP2 as anode for Na/K ion batteries
npj 2D Materials and Applications (2024)
-
Cost and performance analysis as a valuable tool for battery material research
Nature Reviews Materials (2024)
-
Rapid-charging aluminium-sulfur batteries operated at 85 °C with a quaternary molten salt electrolyte
Nature Communications (2024)
-
Carbon-coated hybrid crystals with fast electrochemical reaction kinetics for ultra-stable and high-load sodium-ion batteries
Rare Metals (2024)
-
A Novel NASICON-Type Na3.5MnCr0.5Ti0.5(PO4)3 Nanofiber with Multi-electron Reaction for High-Performance Sodium-Ion Batteries
Advanced Fiber Materials (2024)