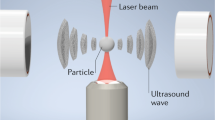
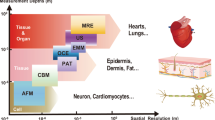
Abstract
Cellular signalling is governed in large part by mechanical forces and electromagnetic fields. Mechanical forces play a critical role in cell differentiation, tissue organization and diseases such as cancer and heart disease; electrical fields are essential for intercellular communication, muscle contraction, neural signalling and sensory perception. Therefore, quantifying a biological system's forces and fields is crucial for understanding physiology and disease pathology and for developing medical tools for repair and recovery. This Review highlights advances in sensing mechanical forces and electrical fields in vivo, focusing on optical probes. The emergence of biocompatible optical probes, such as genetically encoded voltage indicators, molecular rotors, fluorescent dyes, semiconducting nanoparticles, plasmonic nanoparticles and lanthanide-doped upconverting nanoparticles, offers exciting opportunities to push the limits of spatial and temporal resolution, stability, multi-modality and stimuli sensitivity in bioimaging. We further discuss the materials design principles behind these probes and compare them across various metrics to facilitate sensor selection. Finally, we examine which advances are necessary to fully unravel the role of mechanical forces and electrical fields in vivo, such as the ability to probe the vectorial nature of forces, the development of combined force and field sensors, and the design of efficient optical actuators.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Alexander, R. M. The Human Machine (Columbia Univ. Press, 1992).
Autumn, K. et al. Adhesive force of a single gecko foot-hair. Nature 405, 681–685 (2000).
Fisher, M. E. & Kolomeisky, A. B. The force exerted by a molecular motor. Proc. Natl Acad. Sci. USA 96, 6597–6602 (1999).
Cigognini, D. et al. Engineering in vitro microenvironments for cell based therapies and drug discovery. Drug Discov. Today 18, 1099–1108 (2013).
Moulia, B. Plant biomechanics and mechanobiology are convergent paths to flourishing interdisciplinary research. J. Exp. Bot. 64, 4617–4633 (2013).
Xu, W. et al. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE 7, e46609 (2012).
Reardon, S. A giant neuron found wrapped around entire mouse brain. Nature 543, 14–15 (2017).
Sachdev, R. N., Ebner, F. F. & Wilson, C. J. Effect of subthreshold up and down states on the whisker-evoked response in somatosensory cortex. J. Neurophysiol. 92, 3511–3521 (2004).
Zhang, P. C., Keleshian, A. M. & Sachs, F. Voltage-induced membrane movement. Nature 413, 428–432 (2001).
Tyler, W. J. The mechanobiology of brain function. Nat. Rev. Neurosci. 13, 867–878 (2012).
Korneyev, A. Y. Stress-induced tau phosphorylation in mouse strains with different brain Erk1 + 2 immunoreactivity. Neurochem. Res. 23, 1539–1543 (1998).
Style, R. W. et al. Traction force microscopy in physics and biology. Soft Matter 10, 4047–4055 (2014).
Dufrene, Y. F. et al. Imaging modes of atomic force microscopy for application in molecular and cell biology. Nat. Nanotechnol. 12, 295–307 (2017).
Alsteens, D. et al. Atomic force microscopy-based characterization and design of biointerfaces. Nat. Rev. Mater. 2, 17008 (2017).
Fazal, F. M. & Block, S. M. Optical tweezers study life under tension. Nat. Photonics 5, 318–321 (2011).
Molleman, A. Patch Clamping: An Introductory Guide to Patch Clamp Electrophysiology (John Wiley & Sons, 2002).
Brugues, A. et al. Forces driving epithelial wound healing. Nat. Phys. 10, 683–690 (2014).
Koch, T. M., Munster, S., Bonakdar, N., Butler, J. P. & Fabry, B. 3D traction forces in cancer cell invasion. PLoS ONE 7, e33476 (2012).
Mularski, A. et al. Atomic force microscopy reveals the mechanobiology of lytic peptide action on bacteria. Langmuir 31, 6164–6171 (2015).
Benoit, M., Gabriel, D., Gerisch, G. & Gaub, H. E. Discrete interactions in cell adhesion measured by single-molecule force spectroscopy. Nat. Cell Biol. 2, 313–317 (2000).
Finer, J. T., Simmons, R. M. & Spudich, J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368, 113–119 (1994).
Perkins, T. T., Dalal, R. V., Mitsis, P. G. & Block, S. M. Sequence-dependent pausing of single lambda exonuclease molecules. Science 301, 1914–1918 (2003).
Bean, B. P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 8, 451–465 (2007).
Zhang, J., Mehta, S. & Schultz, C. Optical Probes in Biology (CRC Press, 2015).
Cost, A. L., Ringer, P., Chrostek-Grashoff, A. & Grashoff, C. How to measure molecular forces in cells: a guide to evaluating genetically-encoded FRET-based tension sensors. Cell. Mol. Bioeng. 8, 96–105 (2015).
Wegner, K. D. & Hildebrandt, N. Quantum dots: bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem. Soc. Rev. 44, 4792–4834 (2015).
Antaris, A. L. et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 15, 235–242 (2016).
Smith, A. M., Mancini, M. C. & Nie, S. Bioimaging: second window for in vivo imaging. Nat. Nanotechnol. 4, 710–711 (2009).
Chung, K. et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337 (2013).
Russell, E. S. Form and Function: a Contribution to the History of Animal Morphology (J. Murray, 1916).
Roux, W. Gesammelte Abhandlungen über Entwicklungsmechanik der Organismen [German] (Wilhelm Engelmann, 1985).
Fung, Y. C. Biomechanics Mechanical Properties of Living Tissues (Springer, 1981).
Harris, A., Wild, P. & Stopak, D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science 208, 177–179 (1980).
Binnig, G. & Rohrer, H. Scanning tunneling microscopy. Helvet. Phys. Acta 55, 726–735 (1982).
Ashkin, A., Dziedzic, J. M., Bjorkholm, J. E. & Chu, S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt. Lett. 11, 288 (1986).
Freikamp, A., Cost, A. L. & Grashoff, C. The piconewton force awakens: quantifying mechanics in cells. Trends Cell Biol. 26, 838–847 (2016).
Nelson, C. M. From static to animated: measuring mechanical forces in tissues. J. Cell Biol. 216, 29–30 (2017).
Campas, O. A toolbox to explore the mechanics of living embryonic tissues. Semin. Cell Dev. Biol. 55, 119–130 (2016).
Jurchenko, C. & Salaita, K. S. Lighting up the force: investigating mechanisms of mechanotransduction using fluorescent tension probes. Mol. Cell. Biol. 35, 2570–2582 (2015).
Roca-Cusachs, P., Conte, V. & Trepat, X. Quantifying forces in cell biology. Nat. Cell Biol. 19, 742–751 (2017).
Stephens, R. E. Analysis of muscle contraction by ultraviolet microbeam disruption of sarcomere structure. J. Cell Biol. 25, 129–139 (1965).
Soloperto, A. et al. Laser nano-neurosurgery from gentle manipulation to nano-incision of neuronal cells and scaffolds: an advanced neurotechnology tool. Front. Neurosci. 10, 101 (2016).
Sun, Y., Kim, D.-H. & Simmons, C. A. Integrative Mechanobiology: Micro- and Nano- Techniques in Cell Mechanobiology (Cambridge Univ. Press, 2015).
Sweeney, S. T., Hidalgo, A., de Belle, J. S. & Keshishian, H. Embryonic cell ablation in Drosophila using lasers. Cold Spring Harb. Protoc. 2012, 691–693 (2012).
Avery, L. & Horvitz, H. R. A cell that dies during wild-type C. elegans development can function as a neuron in a ced-3 mutant. Cell 51, 1071–1078 (1987).
Behrndt, M. et al. Forces driving epithelial spreading in zebrafish gastrulation. Science 338, 257–260 (2012).
Mondia, J. P., Adams, D. S., Orendorff, R. D., Levin, M. & Omenetto, F. G. Patterned femtosecond-laser ablation of Xenopus laevis melanocytes for studies of cell migration, wound repair, and developmental processes. Biomed. Opt. Express 2, 2383–2391 (2011).
Heller, E., Kumar, K. V., Grill, S. W. & Fuchs, E. Forces generated by cell intercalation tow epidermal sheets in mammalian tissue morphogenesis. Dev. Cell 28, 617–632 (2014).
Yanik, M. F. et al. Neurosurgery: functional regeneration after laser axotomy. Nature 432, 822 (2004).
Upadhyaya, A., Chabot, J. R., Andreeva, A., Samadani, A. & van Oudenaarden, A. Probing polymerization forces by using actin-propelled lipid vesicles. Proc. Natl Acad. Sci. USA 100, 4521–4526 (2003).
Dolega, M. E. et al. Cell-like pressure sensors reveal increase of mechanical stress towards the core of multicellular spheroids under compression. Nat. Commun. 8, 14056 (2017).
Boukellal, H., Campas, O., Joanny, J. F., Prost, J. & Sykes, C. Soft Listeria : actin-based propulsion of liquid drops. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 69, 061906 (2004).
Campas, O. et al. Quantifying cell-generated mechanical forces within living embryonic tissues. Nat. Methods 11, 183–189 (2014). This paper demonstrates the technique of using fluorinated oil droplets to measure the anisotropic forces exerted by cells.
McLure, I. A., Soares, V. A. M. & Edmonds, B. Surface tension of perfluoropropane, perfluoro-n-butane, perfluoro-n-hexane, perfluoro-octane, perfluorotributylamine and n-pentane. Application of the principle of corresponding states to the surface tension of perfluoroalkanes. J. Chem. Soc., Faraday Trans. 1 78, 2251–2257 (1982).
Turansky, R., Konopka, M., Doltsinis, N. L., Stich, I. & Marx, D. Optical, mechanical, and opto-mechanical switching of anchored dithioazobenzene bridges. ChemPhysChem 11, 345–348 (2010).
Kelly, T. R., De Silva, H. & Silva, R. A. Unidirectional rotary motion in a molecular system. Nature 401, 150–152 (1999).
Yang, Q. Z. et al. A molecular force probe. Nat. Nanotechnol. 4, 302–306 (2009).
Kottas, G. S., Clarke, L. I., Horinek, D. & Michl, J. Artificial molecular rotors. Chem. Rev. 105, 1281–1376 (2005).
Jameson, L. P., Balaz, M., Dzyuba, S. V. & Kamiya, N. Conformational preference of a porphyrin rotor in confined environments. RSC Adv. 4, 705–708 (2014).
Kamat, N. P. et al. Sensing membrane stress with near IR-emissive porphyrins. Proc. Natl Acad. Sci. USA 108, 13984–13989 (2011).
Lopez-Duarte, I., Vu, T. T., Izquierdo, M. A., Bull, J. A. & Kuimova, M. K. A molecular rotor for measuring viscosity in plasma membranes of live cells. Chem. Commun. 50, 5282–5284 (2014).
Sherin, P. S. et al. Visualising the membrane viscosity of porcine eye lens cells using molecular rotors. Chem. Sci. 8, 3523–3528 (2017). This paper demonstrates the in vivo use of molecular rotors for studying the local viscosity within a tumour.
Shimolina, L. E. et al. Imaging tumor microscopic viscosity in vivo using molecular rotors. Sci. Rep. 7, 41097 (2017).
Shao, J., Lei, Y., Wen, Z., Dou, Y. & Wang, Z. Nonadiabatic simulation study of photoisomerization of azobenzene: detailed mechanism and load-resisting capacity. J. Chem. Phys. 129, 164111 (2008).
Meng, F., Suchyna, T. M. & Sachs, F. A fluorescence energy transfer-based mechanical stress sensor for specific proteins in situ. FEBS J. 275, 3072–3087 (2008).
Grashoff, C. et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266 (2010). This paper is the first demonstration and calibration of a tethered FRET force sensor, TSmod.
Scholl, J. A. et al. Evolution of plasmonic metamolecule modes in the quantum tunneling regime. ACS Nano 10, 1346–1354 (2016).
Chen, T., Hong, Y. & Reinhard, B. M. Probing DNA stiffness through optical fluctuation analysis of plasmon rulers. Nano Lett. 15, 5349–5357 (2015).
Sonnichsen, C., Reinhard, B. M., Liphardt, J. & Alivisatos, A. P. A molecular ruler based on plasmon coupling of single gold and silver nanoparticles. Nat. Biotechnol. 23, 741–745 (2005).
Xiong, B. et al. Single plasmonic nanosprings for visualizing reactive-oxygen-species-activated localized mechanical force transduction in live cells. ACS Nano 11, 541–548 (2017).
Freikamp, A., Mehlich, A., Klingner, C. & Grashoff, C. Investigating piconewton forces in cells by FRET-based molecular force microscopy. J. Struct. Biol. 197, 37–42 (2017).
Yao, M. et al. The mechanical response of talin. Nat. Commun. 7, 11966 (2016).
Kumar, A. et al. Talin tension sensor reveals novel features of focal adhesion force transmission and mechanosensitivity. J. Cell Biol. 213, 371–383 (2016).
Austen, K. et al. Extracellular rigidity sensing by talin isoform-specific mechanical linkages. Nat. Cell Biol. 17, 1597–1606 (2015).
Paluch, E. K. et al. Mechanotransduction: use the force(s). BMC Biol. 13, 47 (2015).
Fan, X., Zheng, W. & Singh, D. J. Light scattering and surface plasmons on small spherical particles. Light Sci. Appl. 3, e179 (2014).
Scholl, J. A., Koh, A. L. & Dionne, J. A. Quantum plasmon resonances of individual metallic nanoparticles. Nature 483, 421–427 (2012).
Alivisatos, A. P., Gu, W. & Larabell, C. Quantum dots as cellular probes. Annu. Rev. Biomed. Eng. 7, 55–76 (2005).
Larson, D. R. et al. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science 300, 1434–1436 (2003).
Liu, L. et al. Shape control of CdSe nanocrystals with zinc blende structure. J. Am. Chem. Soc. 131, 16423–16429 (2009).
Choi, C. L., Koski, K. J., Sivasankar, S. & Alivisatos, A. P. Strain-dependent photoluminescence behavior of CdSe/CdS nanocrystals with spherical, linear, and branched topologies. Nano Lett. 9, 3544–3549 (2009).
Fang, L. et al. Mechanical and electrical properties of CdTe tetrapods studied by atomic force microscopy. J. Chem. Phys. 127, 184704 (2007). This paper demonstrates the use of quantum dot tetrapods as optical sensors of uniaxial strain acting on polyester fibres in situ.
Choi, C. L., Koski, K. J., Olson, A. C. & Alivisatos, A. P. Luminescent nanocrystal stress gauge. Proc. Natl Acad. Sci. USA 107, 21306–21310 (2010).
Shen, J., Sun, L. D. & Yan, C. H. Luminescent rare earth nanomaterials for bioprobe applications. Dalton Trans. 14, 5687–5697 (2008).
Wisser, M. D. et al. Strain-induced modification of optical selection rules in lanthanide-based upconverting nanoparticles. Nano Lett. 15, 1891–1897 (2015).
Wisser, M. D. et al. Enhancing quantum yield via local symmetry distortion in lanthanide-based upconverting nanoparticles. ACS Photonics 3, 1523–1530 (2016).
Lay, A. et al. Upconverting nanoparticles as optical sensors of nano- to micro-newton forces. Nano Lett. 17, 4172–4177 (2017). This paper demonstrates the calibration and characterization of upconverting nanoparticles as optical sensors of nN to μN forces.
Levy, E. S. et al. Energy-looping nanoparticles: harnessing excited-state absorption for deep-tissue imaging. ACS Nano 10, 8423–8433 (2016).
Nyk, M., Kumar, R., Ohulchanskyy, T. Y., Bergey, E. J. & Prasad, P. N. High contrast in vitro and in vivo photoluminescence bioimaging using near infrared to near infrared up-conversion in Tm3+ and Yb3+ doped fluoride nanophosphors. Nano Lett. 8, 3834–3838 (2008).
Gnach, A., Lipinski, T., Bednarkiewicz, A., Rybka, J. & Capobianco, J. A. Upconverting nanoparticles: assessing the toxicity. Chem. Soc. Rev. 44, 1561–1584 (2015).
Hodgkin, A. L. & Huxley, A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952).
Sakmann, B. & Neher, E. Patch clamp techniques for studying ionic channels in excitable membranes. Annu. Rev. Physiol. 46, 455–472 (1984).
Neher, E. & Sakmann, B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 260, 799–802 (1976).
Campbell, P. K., Jones, K. E., Huber, R. J., Horch, K. W. & Normann, R. A. A silicon-based, three-dimensional neural interface: manufacturing processes for an intracortical electrode array. IEEE Trans. Biomed. Eng. 38, 758–768 (1991).
Park, S. et al. One-step optogenetics with multifunctional flexible polymer fibers. Nat. Neurosci. 20, 612–619 (2017).
Drake, K. L., Wise, K. D., Farraye, J., Anderson, D. J. & BeMent, S. L. Performance of planar multisite microprobes in recording extracellular single-unit intracortical activity. IEEE Trans. Biomed. Eng. 35, 719–732 (1988).
Jones, K. E., Campbell, P. K. & Normann, R. A. A glass/silicon composite intracortical electrode array. Ann. Biomed. Engineer. 20, 423–437 (1992).
Nordhausen, C. T., Rousche, P. J. & Normann, R. A. Optimizing recording capabilities of the Utah Intracortical Electrode Array. Brain Res. 637, 27–36 (1994).
Nordhausen, C. T., Maynard, E. M. & Normann, R. A. Single unit recording capabilities of a 100 microelectrode array. Brain Res. 726, 129–140 (1996).
Chen, R., Canales, A. & Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. 2, 16093 (2017).
Rohatgi, P., Langhals, N. B., Kipke, D. R. & Patil, P. G. In vivo performance of a microelectrode neural probe with integrated drug delivery. Neurosurg. Focus 27, E8 (2009).
Hamel, E. J., Grewe, B. F., Parker, J. G. & Schnitzer, M. J. Cellular level brain imaging in behaving mammals: an engineering approach. Neuron 86, 140–159 (2015).
Peterka, D. S., Takahashi, H. & Yuste, R. Imaging voltage in neurons. Neuron 69, 9–21 (2011).
Akerboom, J. et al. Optimization of a GCaMP calcium indicator for neural activitymaging. J. Neurosci. 32, 13819–13840 (2012).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Podor, B. et al. Comparison of genetically encoded calcium indicators for monitoring action potentials in mammalian brain by two-photon excitation fluorescence microscopy. Neurophotonics 2, 021014 (2015).
Horikawa, K. et al. Spontaneous network activity visualized by ultrasensitive Ca2+ indicators, yellow Cameleon-Nano. Nat. Methods 7, 729–732 (2010).
Lutcke, H. et al. Optical recording of neuronal activity with a genetically-encoded calcium indicator in anesthetized and freely moving mice. Front. Neural Circuits 4, 9 (2010).
Chen, J. L., Carta, S., Soldado-Magraner, J., Schneider, B. L. & Helmchen, F. Behaviour-dependent recruitment of long-range projection neurons in somatosensory cortex. Nature 499, 336–340 (2013).
Zhao, Y. et al. An expanded palette of genetically encoded Ca2+ indicators. Science 333, 1888–1891 (2011).
Helmchen, F., Borst, J. G. & Sakmann, B. Calcium dynamics associated with a single action potential in a CNS presynaptic terminal. Biophys. J. 72, 1458–1471 (1997).
Koester, H. J. & Sakmann, B. Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex. J. Physiol. 529, 625–646 (2000).
Theis, L. et al. Benchmarking spike rate inference in population calciumimaging. Neuron 90, 471–482 (2016).
Tsytsarev, V. et al. Recent progress in voltage-sensitive dye imaging for neuroscience. J. Nanosci. Nanotechnol. 14, 4733–4744 (2014).
Canepari, M. & Zecevic, D. Membrane Potential Imaging in the Nervous System: Methods and Applications (Springer, 2010).
Yan, P. et al. Palette of fluorinated voltage-sensitive hemicyanine dyes. Proc. Natl Acad. Sci. USA 109, 20443–20448 (2012).
Cohen, L. B. et al. Changes in axon fluorescence during activity: molecular probes of membrane potential. J. Membrane Biol. 19, 1–36 (1974).
Palmer, L. M. & Stuart, G. J. Site of action potential initiation in layer 5 pyramidal neurons. J. Neurosci. 26, 1854–1863 (2006).
Lin, M. Z. & Schnitzer, M. J. Genetically encoded indicators of neuronal activity. Nat. Neurosci. 19, 1142–1153 (2016).
Grinvald, A. & Hildesheim, R. VSDI: a new era in functional imaging of cortical dynamics. Nat. Rev. Neurosci. 5, 874–885 (2004).
Brown, C. E., Aminoltejari, K., Erb, H., Winship, I. R. & Murphy, T. H. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J. Neurosci. 29, 1719–1734 (2009).
Ahmed, B. et al. Cortical dynamics subserving visual apparent motion. Cereb. Cortex 18, 2796–2810 (2008).
Chemla, S. & Chavane, F. Voltage-sensitive dye imaging: technique review and models. J. Physiol. Paris 104, 40–50 (2010).
Benucci, A., Frazor, R. A. & Carandini, M. Standing waves and traveling waves distinguish two circuits in visual cortex. Neuron 55, 103–117 (2007).
Slovin, H., Arieli, A., Hildesheim, R. & Grinvald, A. Long-term voltage-sensitive dye imaging reveals cortical dynamics in behaving monkeys. J. Neurophysiol. 88, 3421–3438 (2002). This paper uses the voltage-sensitive dye RH-1691 to visualize neural activity in vivo in monkeys, providing functional maps of the sensory periphery in deep brain structures.
Zhou, W. L., Yan, P., Wuskell, J. P., Loew, L. M. & Antic, S. D. Intracellular long-wavelength voltage-sensitive dyes for studying the dynamics of action potentials in axons and thin dendrites. J. Neurosci. Methods 164, 225–239 (2007).
Foust, A. J., Yu, Y., Popovic, M., Zecevic, D. & McCormick, D. A. Somatic membrane potential and Kv1 channels control spike repolarization in cortical axon collaterals and presynaptic boutons. J. Neurosci. 31, 15490–15498 (2011).
Treger, J. S., Priest, M. F., Iezzi, R. & Bezanilla, F. Real-time imaging of electrical signals with an infrared FDA-approved dye. Biophys. J. 107, L09–12 (2014).
Mutoh, H., Mishina, Y., Gallero-Salas, Y. & Knopfel, T. Comparative performance of a genetically-encoded voltage indicator and a blue voltage sensitive dye for large scale cortical voltage imaging. Front. Cell Neurosci. 9, 147 (2015).
González, J. E. & Tsien, R. Y. Voltage sensing by fluorescence resonance energy transfer in single cells. Biophys. J. 69, 1272–1280 (1995).
Lundby, A., Akemann, W. & Knopfel, T. Biophysical characterization of the fluorescent protein voltage probe VSFP2.3 based on the voltage-sensing domain of Ci-VSP. Eur. Biophys. J. 39, 1625–1635 (2010).
Perron, A. et al. Second and third generation voltage-sensitive fluorescent proteins for monitoring membrane potential. Front. Mol. Neurosci. 2, 5 (2009).
Perron, A., Mutoh, H., Launey, T. & Knopfel, T. Red-shifted voltage-sensitive fluorescent proteins. Chem. Biol. 16, 1268–1277 (2009).
Akemann, W. et al. Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J. Neurophysiol. 108, 2323–2337 (2012).
Barnett, L., Platisa, J., Popovic, M., Pieribone, V. A. & Hughes, T. A fluorescent, genetically-encoded voltage probe capable of resolving action potentials. PLoS ONE 7, e43454 (2012).
Jin, L. et al. Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron 75, 779–785 (2012).
St-Pierre, F. et al. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat. Neurosci. 17, 884–889 (2014).
Gong, Y. et al. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science 350, 1361–1366 (2015). This paper presents a FRET-based GEVI that is sufficiently bright and fast to resolve fast spike trains with low detection error rates for in vivo imaging of sensory-evoked responses in mice and flies.
Gong, Y., Wagner, M. J., Zhong Li, J. & Schnitzer, M. J. Imaging neural spiking in brain tissue using FRET-opsin protein voltage sensors. Nat. Commun. 5, 3674 (2014).
Miller, E. W. Small molecule fluorescent voltage indicators for studying membrane potential. Curr. Opin. Chem. Biol. 33, 74–80 (2016).
Woodford, C. R. et al. Improved PeT molecules for optically sensing voltage in neurons. J. Am. Chem. Soc. 137, 1817–1824 (2015).
Kulkarni, R. U. et al. A rationally designed, general strategy for membrane orientation of photoinduced electron transfer-based voltage-sensitive dyes. ACS Chem. Biol. 12, 407–413 (2017). This paper uses molecular dynamics simulations to guide the design of PeT-based voltage-sensitive dyes, demonstrating nine new dyes with an optimized alignment with respect to the cell membrane, which makes them the most sensitive PeT-based dyes to date.
Yang, H. H. et al. Subcellular imaging of voltage and calcium signals reveals neural processing in vivo. Cell 166, 245–257 (2016).
Molokanova, E. B. et al. Quantum dots move beyond fluorescence imaging. Biophotonics Int. 6, 26–31 (2008).
Marshall, J. D. & Schnitzer, M. J. Optical strategies for sensing neuronal voltage using quantum dots and other semiconductor nanocrystals. ACS Nano 7, 4601–4609 (2013).
Rowland, C. E. et al. Electric field modulation of semiconductor quantum dot photoluminescence: insights into the design of robust voltage-sensitive cellular imaging probes. Nano Lett. 15, 6848–6854 (2015). This paper demonstrates the potential of quantum dots to track action potential firing with a millisecond time resolution.
Nag, O. K. et al. Quantum dot-peptide-fullerene bioconjugates for visualization of in vitro and in vivo cellular membrane potential. ACS Nano 11, 5598–5613 (2017).
Choi, H. S. et al. Renal clearance of quantum dots. Nat. Biotechnol. 25, 1165–1170 (2007).
Zhu, Y., Hong, H., Xu, Z., Li, Z. & Cai, W. Quantum dot-based nanoprobes for in vivo targeted imaging. Curr. Mol. Med. 13, 1549–1567 (2013).
Zhelev, Z., Ohba, H. & Bakalova, R. Single quantum dot-micelles coated with silica shell as potentially non-cytotoxic fluorescent cell tracers. J. Am. Chem. Soc. 128, 6324–6325 (2006).
Hall, L. T. et al. High spatial and temporal resolution wide-field imaging of neuron activity using quantum NV-diamond. Sci. Rep. 2, 401 (2012).
Boretti, A. & Castelletto, S. Nanometric resolution magnetic resonance imaging methods for mapping functional activity in neuronal networks. MethodsX 3, 297–306 (2016).
Chen, E. H. et al. High-sensitivity spin-based electrometry with an ensemble of nitrogen-vacancy centers in diamond. Phys. Rev. A 95, 053417 (2017).
Barson, M. S. et al. Nanomechanical sensing using spins in diamond. Nano Lett. 17, 1496–1503 (2017).
Montalti, M., Cantelli, A. & Battistelli, G. Nanodiamonds and silicon quantum dots: ultrastable and biocompatible luminescent nanoprobes for long-term bioimaging. Chem. Soc. Rev. 44, 4853–4921 (2015).
Mohan, N. & Chang, H.-C. in Optical Engineering of Diamond (eds Mildren, R. P. & Rabeau, J. R. ) 445–471 (Wiley, 2013).
Barry, J. F. et al. Optical magnetic detection of single-neuron action potentials using quantum defects in diamond. Proc. Natl Acad. Sci. USA 113, 14133–14138 (2016). This paper demonstrates the ability of nitrogen vacancy centres to detect single action potentials in living worms with no adverse effects on the animal for measurements longer than 24 hours.
Wee, T. L. et al. Two-photon excited fluorescence of nitrogen-vacancy centers in proton-irradiated type Ib diamond. J. Phys. Chem. A 111, 9379–9386 (2007).
Hui, Y. Y. et al. Two-photon fluorescence correlation spectroscopy of lipid-encapsulated fluorescent nanodiamonds in living cells. Opt. Express 18, 5896–5905 (2010).
Neburkova, J., Vavra, J. & Cigler, P. Coating nanodiamonds with biocompatible shells for applications in biology and medicine. Curr. Opin. Solid State Mater. Sci. 21, 43–53 (2017).
Akerman, M. E., Chan, W. C., Laakkonen, P., Bhatia, S. N. & Ruoslahti, E. Nanocrystal targeting in vivo. Proc. Natl Acad. Sci. USA 99, 12617–12621 (2002).
Frangioni, J. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 7, 626–634 (2003).
Miyamoto, K., Refojo, M. F., Tolentino, F. I., Fournier, G. A. & Albert, D. M. Fluorinated oils as experimental vitreous substitutes. Arch. Ophthalmol. 104, 1053–1056 (1986).
Browning, L. M., Huang, T. & Xu, X. H. Real-time in vivo imaging of size-dependent transport and toxicity of gold nanoparticles in zebrafish embryos using single nanoparticle plasmonic spectroscopy. Interface Focus 3, 20120098 (2013).
Yang, H. H. & St-Pierre, F. Genetically encoded voltage indicators: opportunities and challenges. J. Neurosci. 36, 9977–9989 (2016).
Murphy, C. J. et al. Gold nanoparticles in biology: beyond toxicity to cellularimaging. Acc. Chem. Res. 41, 1721–1730 (2008).
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic. Bioengineer. Transl Med. 1, 10–29 (2016).
Ashman, R. B., Rho, J. Y. & Turner, C. H. Anatomical variation of orthotropic elastic moduli of the proximal human tibia. J. Biomechan. 22, 895–900 (1989).
Gennisson, J. L. et al. Viscoelastic and anisotropic mechanical properties of in vivo muscle tissue assessed by supersonic shearimaging. Ultrasound Med. Biol. 36, 789–801 (2010).
Fink, J. et al. External forces control mitotic spindle positioning. Nat. Cell Biol. 13, 771–778 (2011).
Stuart, G., Spruston, N., Sakmann, B. & Häusser, M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 20, 125–131 (1997).
Yu, Y., Shu, Y. & McCormick, D. A. Cortical action potential backpropagation explains spike threshold variability and rapid-onset kinetics. J. Neurosci. 28, 7260–7272 (2008).
Holthoff, K., Zecevic, D. & Konnerth, A. Rapid time course of action potentials in spines and remote dendrites of mouse visual cortex neurons. J. Physiol. 588, 1085–1096 (2010).
Liu, D. et al. Three-dimensional controlled growth of monodisperse sub-50 nm heterogeneous nanocrystals. Nat. Commun. 7, 10254 (2016).
Raja, S. N. et al. Tetrapod nanocrystals as fluorescent stress probes of electrospun nanocomposites. Nano Lett. 13, 3915–3922 (2013).
Medvedev, M. G., Bushmarinov, I. S., Sun, J., Perdew, J. P. & Lyssenko, K. A. Density functional theory is straying from the path toward the exact functional. Science 355, 49–52 (2017).
Polking, M. J., Alivisatos, A. P. & Ramesh, R. Synthesis, physics, and applications of ferroelectric nanomaterials. MRS Commun. 5, 27–44 (2015).
Yin, A. et al. Plasmonic/nonlinear optical material core/shell nanorods as nanoscale plasmon modulators and optical voltage sensors. Angew. Chem. Int. Ed Engl. 55, 583–587 (2016).
Liu, S., Borys, N. J., Huang, J., Talapin, D. V. & Lupton, J. M. Exciton storage in CdSe/CdS tetrapod semiconductor nanocrystals: electric field effects on exciton and multiexciton states. Phys. Rev. B 86, 045303 (2012).
Svechtarova, M. I. et al. Sensor devices inspired by the five senses: a review. Electroanalysis 28, 1201–1241 (2016).
Valon, L., Marin-Llaurado, A., Wyatt, T., Charras, G. & Trepat, X. Optogenetic control of cellular forces and mechanotransduction. Nat. Commun. 8, 14396 (2017).
Liu, Z. et al. Nanoscale optomechanical actuators for controlling mechanotransduction in living cells. Nat. Methods 13, 143–146 (2016).
Seo, D. et al. A mechanogenetic toolkit for interrogating cell signaling in space and time. Cell 165, 1507–1518 (2016).
Kim, C. K., Adhikari, A. & Deisseroth, K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat. Rev. Neurosci. 18, 222–235 (2017).
Fenno, L., Yizhar, O. & Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 34, 389–412 (2011).
Brinks, D., Adam, Y., Kheifets, S. & Cohen, A. E. Painting with rainbows: patterning light in space, time, and wavelength for multiphoton optogenetic sensing and control. Acc. Chem. Res. 49, 2518–2526 (2016).
Marino, A. et al. Piezoelectric nanoparticle-assisted wireless neuronal stimulation. ACS Nano 9, 7678–7689 (2015).
Guduru, R. et al. Magnetoelectric ‘spin’ on stimulating the brain. Nanomedicine 10, 2051–2061 (2015).
Chen, R., Romero, G., Christiansen, M. G., Mohr, A. & Anikeeva, P. Wireless magnetothermal deep brain stimulation. Science 347, 1477–1480 (2015).
Kim, J. W. et al. Single-cell mechanogenetics using monovalent magnetoplasmonic nanoparticles. Nat. Protoc. 12, 1871–1889 (2017).
Lugo, K., Miao, X., Rieke, F. & Lin, L. Y. Remote switching of cellular activity and cell signaling using light in conjunction with quantum dots. Biomed. Opt. Express 3, 447–454 (2012).
Förster, T. Zwischenmolekulare Energiewanderung und Fluoreszenz [German]. Annalen Physik 437, 55–75 (1948).
Beardsley, K. & Cantor, C. R. Studies of transfer RNA tertiary structure by singlet-singlet energy transfer. Proc. Natl Acad. Sci. USA 65, 39–46 (1970).
Stryer, L. Fluorescence energy transfer as a spectroscopic ruler. Annu. Rev. Biochem. 47, 819–846 (1978).
Jares-Erijman, E. A. & Jovin, T. M. FRET imaging. Nat. Biotechnol. 21, 1387–1395 (2003).
Lam, A. J. et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat. Methods 9, 1005–1012 (2012).
Zhang, C., Wei, Z. H. & Ye, B. C. Imaging and tracing of intracellular metabolites utilizing genetically encoded fluorescent biosensors. Biotechnol. J. 8, 1280–1291 (2013).
Gayrard, C. & Borghi, N. FRET-based molecular tension microscopy. Methods 94, 33–42 (2016).
Miessler, G. L., Fischer, P. J. & Tarr, D. A. Inorganic Chemistry 5th edn (Pearson, 2013).
Werts, M. H. Making sense of lanthanide luminescence. Sci. Prog. 88, 101–131 (2005).
Meng, F., Suchyna, T. M., Lazakovitch, E., Gronostajski, R. M. & Sachs, F. Real time FRET based detection of mechanical stress in cytoskeletal and extracellular matrix proteins. Cell. Mol. Bioeng. 4, 148–159 (2011).
Akerboom, J. et al. Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J. Biol. Chem. 284, 6455–6464 (2008).
Inagaki, S. et al. Genetically encoded bioluminescent voltage indicator for multi-purpose use in wide range of bioimaging. Sci. Rep. 7, 42398 (2017).
Edwards, J. S., Chen, S. W. & Berns, M. W. Cercal sensory development following laser microlesions of embryonic apical cells in Acheta domesticus. J. Neurosci. 1, 250–258 (1981).
Brugues, J., Nuzzo, V., Mazur, E. & Needleman, D. J. Nucleation and transport organize microtubules in metaphase spindles. Cell 149, 554–564 (2012).
Lau, K. et al. Anisotropic stress orients remodelling of mammalian limb bud ectoderm. Nat. Cell Biol. 17, 569–579 (2015).
Serwane, F. et al. In vivo quantification of spatially varying mechanical properties in developing tissues. Nat. Methods 14, 181–186 (2017).
Kuriyama, S. et al. In vivo collective cell migration requires an LPAR2-dependent increase in tissue fluidity. J. Cell Biol. 206, 113–127 (2014).
Cai, D. et al. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell 157, 1146–1159 (2014).
Krieg, M., Dunn, A. R. & Goodman, M. B. Mechanical control of the sense of touch by β-spectrin. Nat. Cell Biol. 16, 224–233 (2014).
Yamashita, S., Tsuboi, T., Ishinabe, N., Kitaguchi, T. & Michiue, T. Wide and high resolution tension measurement using FRET in embryo. Sci. Rep. 6, 28535 (2016).
Morimatsu, M., Mekhdjian, A. H., Adhikari, A. S. & Dunn, A. R. Molecular tension sensors report forces generated by single integrin molecules in living cells. Nano Lett. 13, 3985–3989 (2013).
Fischer, T. et al. Single semiconductor nanocrystals under compressive stress: reversible tuning of the emission energy. Nano Lett. 17, 1559–1563 (2017).
Bindocci, E. et al. Three-dimensional Ca2+ imaging advances understanding of astrocyte biology. Science 356, eaai8185 (2017).
Sadakane, O. et al. Long-term two-photon calcium imaging of neuronal populations with subcellular resolution in adult non-human primates. Cell Rep. 13, 1989–1999 (2015).
Ahrens, M. B. et al. Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature 485, 471–477 (2012).
Gore, B. B., Soden, M. E. & Zweifel, L. S. Visualization of plasticity in fear-evoked calcium signals in midbrain dopamine neurons. Learn. Mem. 21, 575–579 (2014).
Szalay, G. et al. Fast 3D imaging of spine, dendritic, and neuronal assemblies in behaving animals. Neuron 92, 723–738 (2016).
Tang, Q. et al. In vivo voltage-sensitive dye imaging of subcortical brain function. Sci. Rep. 5, 17325 (2015).
Kuhn, B., Denk, W. & Bruno, R. M. In vivo two-photon voltage-sensitive dye imaging reveals top-down control of cortical layers 1 and 2 during wakefulness. Proc. Natl Acad. Sci. USA 105, 7588–7593 (2008).
Han, J. et al. In vivo voltage-sensitive dye imaging of the insular cortex in nerve-injured rats. Neurosci. Lett. 634, 146–152 (2016).
Tsutsui, H. et al. Improved detection of electrical activity with a voltage probe based on a voltage-sensing phosphatase. J. Physiol. 591, 4427–4437 (2013).
Zou, P. et al. Bright and fast multicoloured voltage reporters via electrochromic FRET. Nat. Commun. 5, 4625 (2014).
Park, K., Deutsch, Z., Li, J. J., Oron, D. & Weiss, S. Single molecule quantum-confined Stark effect measurements of semiconductor nanoparticles at room temperature. ACS Nano 6, 10013–10023 (2012).
Karaveli, S. et al. in 2015 Conference on Lasers and Electro-Optics (CLEO)http://dx.doi.org/10.1364/CLEO_QELS.2015.FTh3B.6 (San Jose, CA, 2015).
Acknowledgements
The authors acknowledge financial support from Stanford NeuroFab and Bio-X Interdisciplinary Initiatives Committee (IIP). R.K. was supported by an Eastman Kodak fellowship. A.L. was supported by the NSF GRFP (2013156180).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Mehlenbacher, R., Kolbl, R., Lay, A. et al. Nanomaterials for in vivo imaging of mechanical forces and electrical fields. Nat Rev Mater 3, 17080 (2018). https://doi.org/10.1038/natrevmats.2017.80
Published:
DOI: https://doi.org/10.1038/natrevmats.2017.80
This article is cited by
-
How multiscale curvature couples forces to cellular functions
Nature Reviews Physics (2024)
-
Fluorescence-amplified nanocrystals in the second near-infrared window for in vivo real-time dynamic multiplexed imaging
Nature Nanotechnology (2023)
-
Versioning biological cells for trustworthy cell engineering
Nature Communications (2022)
-
Time for NanoNeuro
Nature Methods (2021)
-
Measuring mechanical stress in living tissues
Nature Reviews Physics (2020)