Abstract
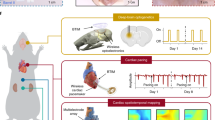
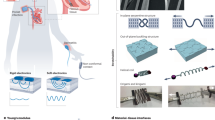
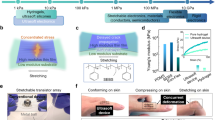
Biomedical electronic devices are interfaced with the human body to extract precise medical data and to interfere with tissue function by providing electrical stimuli. In this Review, we outline physiologically and pathologically relevant tissue properties and processes that are important for designing implantable electronic devices. We summarize design principles for flexible and stretchable electronics that adapt to the mechanics of soft tissues, such as those including conducting polymers, liquid metal alloys, metallic buckling and meandering architectures. We further discuss technologies for inserting devices into the body in a minimally invasive manner and for eliminating them without further intervention. Finally, we introduce the concept of integrating electronic devices with biomaterials and cells, and we envision how such technologies may lead to the development of bionic organs for regenerative medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aquilina, O. A brief history of cardiac pacing. Images Paediatr. Cardiol. 27, 17–81 (2006).
Morton, P. G. Rate-responsive cardiac pacemakers. AACN Adv. Crit. Care 2, 140–149 (1991).
Tomaske, M. et al. A 12-year experience of bipolar steroid-eluting epicardial pacing leads in children. Ann. Thorac. Surg. 85, 1694–1701 (2008).
Cohen, M. I. et al. Permanent epicardial pacing in pediatric patients. Circulation 103, 2585–2590 (2001).
Zeng, F.-G. & Fay, R. R. Cochlear Implants: Auditory Prostheses and Electric Hearing 1–13 (Springer, 2004).
Zeng, F.-G. & Fay, R. R. Cochlear Implants: Auditory prostheses and Electric Hearing (Springer, 2013).
Wilson, B. S. & Dorman, M. F. Cochlear implants: current designs and future possibilities. J. Rehabil. Res. Dev. 45, 695 (2008).
Lacour, S. P., Courtine, G. & Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 1, 15963 (2016).
Fleischer, S. & Dvir, T. Tissue engineering on the nanoscale: lessons from the heart. Curr. Opin. Biotechnol. 24, 664–671 (2013).
Allen, D. & Kurihara, S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J. Physiol. 327, 79 (1982).
Carlsson, M. et al. Total heart volume variation throughout the cardiac cycle in humans. Am. J. Physiol. Heart Circ. Physiol. 287, H243–H250 (2004).
Close, R. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 52, 129–197 (1972).
Gayer, C. P. & Basson, M. D. The effects of mechanical forces on intestinal physiology and pathology. Cell Signal. 21, 1237–1244 (2009).
Scholten, K. & Meng, E. Materials for microfabricated implantable devices: a review. Lab Chip 15, 4256–4272 (2015).
McKee, C. T., Last, J. A., Russell, P. & Murphy, C. J. Indentation versus tensile measurements of Young's modulus for soft biological tissues. Tissue Eng. Part B Rev. 17, 154–163 (2011).
Hiesinger, W. et al. Myocardial tissue elastic properties determined by atomic force microscopy after stromal cell–derived factor 1α angiogenic therapy for acute myocardial infarction in a murine model. J. Thorac. Cardiovasc. Surg. 143, 962–966 (2012).
Discher, D. & Engler, A. in ASME 2007 Summer Bioengineering Conference (ed Steinman, D. A. ) 249–250 (Keystone, 2007).
Engler, A. J. et al. Myotubes differentiate optimally on substrates with tissue-like stiffness. J. Cell Biol. 165, 877–887 (2004).
Goffin, J. M. et al. Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. J. Cell Biol. 171, 259–268 (2006).
Samani, A., Zubovits, J. & Plewes, D. Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 168 samples. Phys. Med. Biol. 52, 1555 (2007).
Wuerfel, J. et al. MR-elastography reveals degradation of tissue integrity in multiple sclerosis. Neuroimage 49, 2520–2525 (2010).
Rowland, L. P. & Shneider, N. A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 344, 1678–1690 (2001).
Discher, D. E., Janmey, P. & Wang, Y.-l. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
Anderson, J. M., Rodriguez, A. & Chang, D. T. Foreign body reaction to biomaterials. Semin. Immunol. 20, 86–100 (2008).
Wilson, C. J., Clegg, R. E., Leavesley, D. I. & Pearcy, M. J. Mediation of biomaterial–cell interactions by adsorbed proteins: a review. Tissue Eng. 11, 1–18 (2005).
Henson, P. M. The immunologic release of constituents from neutrophil leukocytes I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J. Immunol. 107, 1535–1546 (1971).
Christenson, E. M., Anderson, J. M. & Hiltner, A. Oxidative mechanisms of poly (carbonate urethane) and poly (ether urethane) biodegradation: in vivo and in vitro correlations. J. Biomed. Mater. Res. Part A 70, 245–255 (2004).
Brodbeck, W. G. et al. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proc. Natl Acad. Sci. USA 99, 10287–10292 (2002).
Costerton, J., Montanaro, L. & Arciola, C. Biofilm in implant infections: its production and regulation. Int. J. Artif. Organs 28, 1062–1068 (2005).
Fishbein, M. C., Maclean, D. & Maroko, P. R. The histopathologic evolution of myocardial infarction. Chest 73, 843–849 (1978).
Polikov, V. S., Tresco, P. A. & Reichert, W. M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 148, 1–18 (2005).
Pachter, J. S., De Vries, H. E. & Fabry, Z. The blood-brain barrier and its role in immune privilege in the central nervous system. J. Neuropathol. Exp. Neurol. 62, 593–604 (2003).
Chen, R., Canales, A. & Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. 2, 16093 (2017).
Gerweck, L. E. & Seetharaman, K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 56, 1194–1198 (1996).
Xin, Y., Huo, K., Tao, H., Tang, G. & Chu, P. K. Influence of aggressive ions on the degradation behavior of biomedical magnesium alloy in physiological environment. Acta Biomater. 4, 2008–2015 (2008).
Xu, W. et al. Increased production of reactive oxygen species contributes to motor neuron death in a compression mouse model of spinal cord injury. Spinal Cord 43, 204–213 (2005).
Christenson, E. M., Dadsetan, M., Wiggins, M., Anderson, J. M. & Hiltner, A. Poly (carbonate urethane) and poly (ether urethane) biodegradation: in vivo studies. J. Biomed. Mater. Res. Part A 69, 407–416 (2004).
Ali, S., Zhong, S.-P., Doherty, P. & Williams, D. Mechanisms of polymer degradation in implantable devices: I. Poly (caprolactone). Biomaterials 14, 648–656 (1993).
Ali, S., Doherty, P. & Williams, D. Mechanisms of polymer degradation in implantable devices. 2. Poly (DL-lactic acid). J. Biomed. Mater. Res. Part A 27, 1409–1418 (1993).
Omens, J. H. Stress and strain as regulators of myocardial growth. Prog. Biophys. Mol. Biol. 69, 559–572 (1998).
Buchko, C. J., Slattery, M. J., Kozloff, K. M. & Martin, D. C. Mechanical properties of biocompatible protein polymer thin films. J. Mater. Res. 15, 231–242 (2000).
Garnier, F., Hajlaoui, R., Yassar, A. & Srivastava, P. All-polymer field-effect transistor realized by printing techniques. Science 265, 1674–1677 (1994).
McCoul, D., Hu, W., Gao, M., Mehta, V. & Pei, Q. Recent advances in stretchable and transparent electronic materials. Adv. Electron. Mater. 2, 1500407 (2016).
Wang, C., Zheng, W., Yue, Z., Too, C. O. & Wallace, G. G. Buckled, stretchable polypyrrole electrodes for battery applications. Adv. Mater. 23, 3580–3584 (2011).
Yuan, W. et al. in The 14th International Symposium on: Smart Structures and Materials & Nondestructive Evaluation and Health Monitoring 65240N-65240N-65212 (International Society for Optics and Photonics, 2007).
Hansen, T. S., West, K., Hassager, O. & Larsen, N. B. Highly stretchable and conductive polymer material made from poly (3, 4-ethylenedioxythiophene) and polyurethane elastomers. Adv. Funct. Mater. 17, 3069–3073 (2007).
Samba, R., Herrmann, T. & Zeck, G. PEDOT–CNT coated electrodes stimulate retinal neurons at low voltage amplitudes and low charge densities. J. Neural Eng. 12, 015914 (2015).
Owens, R. M. & Malliaras, G. G. Organic electronics at the interface with biology. MRS Bull. 35, 449–456 (2010).
Park, J. et al. Electromechanical cardioplasty using a wrapped elasto-conductive epicardial mesh. Sci. Transl Med. 8, 344ra86 (2016).
Carpi, F., De Rossi, D., Kornbluh, R., Pelrine, R. E. & Sommer-Larsen, P. Dielectric Elastomers as Electromechanical Transducers: Fundamentals, Materials, Devices, Models and Applications of an Emerging Electroactive Polymer Technology. (Elsevier, 2011).
Hu, L., Yuan, W., Brochu, P., Gruner, G. & Pei, Q. Highly stretchable, conductive, and transparent nanotube thin films. Appl. Phys. Lett. 94, 160108 (2009).
Smart, S., Cassady, A., Lu, G. & Martin, D. The biocompatibility of carbon nanotubes. Carbon 44, 1034–1047 (2006).
Tian, F., Cui, D., Schwarz, H., Estrada, G. G. & Kobayashi, H. Cytotoxicity of single-wall carbon nanotubes on human fibroblasts. Toxicol. In Vitro 20, 1202–1212 (2006).
Alarifi, S., Ali, D., Verma, A., Almajhdi, F. N. & Al-Qahtani, A. A. Single-walled carbon nanotubes induce cytotoxicity and DNA damage via reactive oxygen species in human hepatocarcinoma cells. In Vitro Cell. Dev. Biol. Anim. 50, 714–722 (2014).
Dong, J. & Ma, Q. Advances in mechanisms and signaling pathways of carbon nanotube toxicity. Nanotoxicology 9, 658–676 (2015).
Kim, H.-J., Son, C. & Ziaie, B. A multiaxial stretchable interconnect using liquid-alloy-filled elastomeric microchannels. Appl. Phys. Lett. 92, 011904 (2008).
Siegel, A. C., Bruzewicz, D. A., Weibel, D. B. & Whitesides, G. M. Microsolidics: fabrication of three-dimensional metallic microstructures in poly (dimethylsiloxane). Adv. Mater. 19, 727–733 (2007).
Dickey, M. D. et al. Eutectic gallium-indium (EGaIn): a liquid metal alloy for the formation of stable structures in microchannels at room temperature. Adv. Funct. Mater. 18, 1097–1104 (2008).
Khondoker, M. & Sameoto, D. Fabrication methods and applications of microstructured gallium based liquid metal alloys. Smart Mater. Struct. 25, 093001 (2016).
So, J. H. et al. Reversibly deformable and mechanically tunable fluidic antennas. Adv. Funct. Mater. 19, 3632–3637 (2009).
Lide, D. R. Handbook of Chemistry and Physics 88th edn (CRC Press, 2007).
Chiechi, R. C., Weiss, E. A., Dickey, M. D. & Whitesides, G. M. Eutectic gallium–indium (EGaIn): a moldable liquid metal for electrical characterization of self-assembled monolayers. Angew. Chem. Int. Ed. 120, 148–150 (2008).
Jones, J., Lacour, S. P., Wagner, S. & Suo, Z. Stretchable wavy metal interconnects. J. Vac. Sci. Technol. A Vac. Surf. Films 22, 1713–1715 (2004).
Lacour, S. P., Jones, J., Suo, Z. & Wagner, S. Design and performance of thin metal film interconnects for skin-like electronic circuits. IEEE Electron. Device Lett. 25, 178–180 (2004).
Chou, N., Yoo, S. & Kim, S. A largely deformable surface type neural electrode array based on PDMS. IEEE Trans. Neural Syst. Rehabil. Eng. 21, 544–553 (2013).
Graudejus, O., Yu, Z., Jones, J., Morrison, B. & Wagner, S. Characterization of an elastically stretchable microelectrode array and its application to neural field potential recordings. J. Electrochem. Soc. 155, P85–P94 (2009).
Sun, Y., Choi, W. M., Jiang, H., Huang, Y. Y. & Rogers, J. A. Controlled buckling of semiconductor nanoribbons for stretchable electronics. Nat. Nanotechnol. 1, 201–207 (2006).
Khang, D.-Y., Jiang, H., Huang, Y. & Rogers, J. A. A stretchable form of single-crystal silicon for high-performance electronics on rubber substrates. Science 311, 208–212 (2006).
Park, S. I. et al. Theoretical and experimental studies of bending of inorganic electronic materials on plastic substrates. Adv. Funct. Mater. 18, 2673–2684 (2008).
Xu, S. et al. Assembly of micro/nanomaterials into complex, three-dimensional architectures by compressive buckling. Science 347, 154–158 (2015). In this article, a method for creating stretchable 3D structures is presented. These structures could be used to create stretchable flexible 3D electronics.
Xu, J. et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 355, 59–64 (2017).
Oh, J. Y. et al. Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 539, 411–415 (2016).
Lide, D. R. Handbook of Chemistry and Physics 86th edn (CRC Press, 2005).
Gonzalez, M. et al. in EuroSimE 2007 — International Conference on Thermal, Mechanical and Multi-Physics Simulation Experiments in Microelectronics and Micro-Systems PID83 (London, 2007).
Gonzalez, M. et al. Design and performance of metal conductors for stretchable electronic circuits. Circuit World 35, 22–29 (2009).
Fan, J. A. et al. Fractal design concepts for stretchable electronics. Nat. Commun. 5, 3266 (2014).
Kim, D. H. et al. Epidermal electronics. Science 333, 838–843 (2011).
Xu, L. et al. 3D multifunctional integumentary membranes for spatiotemporal cardiac measurements and stimulation across the entire epicardium. Nat. Commun. 5, 3329 (2014).
Kim, D.-H. et al. Materials for multifunctional balloon catheters with capabilities in cardiac electrophysiological mapping and ablation therapy. Nat. Mater. 10, 316–323 (2011).
Song, Y. M. et al. Digital cameras with designs inspired by the arthropod eye. Nature 497, 95–99 (2013).
Huang, X. et al. Stretchable, wireless sensors and functional substrates for epidermal characterization of sweat. Small 10, 3083–3090 (2014).
Webb, R. C. et al. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 12, 938–944 (2013).
Norton, J. J. et al. Soft, curved electrode systems capable of integration on the auricle as a persistent brain-computer interface. Proc. Natl Acad. Sci. USA 112, 3920–3925 (2015).
Bareket, L. et al. Temporary-tattoo for long-term high fidelity biopotential recordings. Sci. Rep. 6, 25727 (2016).
Kim, D.-H. et al. Electronic sensor and actuator webs for large-area complex geometry cardiac mapping and therapy. Proc. Natl Acad. Sci. USA 109, 19910–19915 (2012). This article presents an example of how flexible electronics can interact with internal organs and how they are fabricated so that they adapt to the organ topography.
Chung, H. J. et al. Stretchable, multiplexed pH sensors with demonstrations on rabbit and human hearts undergoing ischemia. Adv. Healthcare Mater. 3, 59–68 (2014).
Lendlein, A. & Langer, R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 296, 1663–1666 (2002).
Soppimath, K. S., Aminabhavi, T. M., Kulkarni, A. R. & Rudzinski, W. E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 70, 1–20 (2001).
Miyamoto, H. et al. Biodegradable scleral implant for intravitreal controlled release of fluconazole. Curr. Res. 16, 930–935 (1997).
Venkatraman, S. S., Tan, L. P., Joso, J. F. D., Boey, Y. C. F. & Wang, X. Biodegradable stents with elastic memory. Biomaterials 27, 1563–1568 (2006).
Hermawan, H., Dubé, D. & Mantovani, D. Developments in metallic biodegradable stents. Acta Biomater. 6, 1683–1687 (2010).
Eppley, B. L. Use of resorbable plates and screws in pediatric facial fractures. J. Oral Maxillofacial Surg. 63, 385–391 (2005).
Irimia-Vladu, M. et al. Biocompatible and biodegradable materials for organic field-effect transistors. Adv. Funct. Mater. 20, 4069–4076 (2010).
Bettinger, C. J. & Bao, Z. Organic thin-film transistors fabricated on resorbable biomaterial substrates. Adv. Mater. 22, 651–655 (2010).
Fu, K. et al. Transient rechargeable batteries triggered by cascade reactions. Nano Lett. 15, 4664–4671 (2015).
Yin, L. et al. Materials, designs, and operational characteristics for fully biodegradable primary batteries. Adv. Mater. 26, 3879–3884 (2014).
Bae, H. et al. Physically transient memory on a rapidly dissoluble paper for security application. Sci. Rep. 6, 38324 (2016).
Hwang, S. W. et al. A physically transient form of silicon electronics. Science 337, 1630–1634 (2012). This article presents a shift in bioresorbable electronics in which silicon-based circuit components can be manufactured to have a transient lifetime.
Yin, L. et al. Dissolvable metals for transient electronics. Adv. Funct. Mater. 24, 645–658 (2014).
Kim, D.-H. et al. Silicon electronics on silk as a path to bioresorbable, implantable devices. Appl. Phys. Lett. 95, 133701 (2009).
Wu, F. et al. Silk-backed structural optimization of high-density flexible intracortical neural probes. J. Microelectromechan. Syst. 24, 62–69 (2015).
Tao, H. et al. Silk-based resorbable electronic devices for remotely controlled therapy and in vivo infection abatement. Proc. Natl Acad. Sci. USA 111, 17285–17289 (2014).
Jin, H. J. et al. Water-stable silk films with reduced β-sheet content. Adv. Funct. Mater. 15, 1241–1247 (2005).
Hwang, S. W. et al. Materials for bioresorbable radio frequency electronics. Adv. Mater. 25, 3526–3531 (2013).
Kim, D.-H. et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 9, 511–517 (2010).
Makadia, H. K. & Siegel, S. J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 3, 1377–1397 (2011).
Acar, H. et al. Study of physically transient insulating materials as a potential platform for transient electronics and bioelectronics. Adv. Funct. Mater. 24, 4135–4143 (2014).
Hwang, S.-W. et al. Biodegradable elastomers and silicon nanomembranes/nanoribbons for stretchable, transient electronics, and biosensors. Nano Lett. 15, 2801–2808 (2015).
Martin, C., Dejardin, T., Hart, A., Riehle, M. O. & Cumming, D. R. Directed nerve regeneration enabled by wirelessly powered electrodes printed on a biodegradable polymer. Adv. Healthcare Mater. 3, 1001–1006 (2014).
Hwang, S. W. et al. High-performance biodegradable/transient electronics on biodegradable polymers. Adv. Mater. 26, 3905–3911 (2014).
Irimia-Vladu, M., Głowacki, E. D., Voss, G., Bauer, S. & Sariciftci, N. S. Green and biodegradable electronics. Mater. Today 15, 340–346 (2012).
Yin, L. et al. Mechanisms for hydrolysis of silicon nanomembranes as used in bioresorbable electronics. Adv. Mater. 27, 1847–1854 (2015).
Lee, G. et al. in ECS Meeting Abstracts 77 (The Electrochemical Society, 2017).
Hwang, S. W. et al. Materials and fabrication processes for transient and bioresorbable high-performance electronics. Adv. Funct. Mater. 23, 4087–4093 (2013).
Yu, K. J. et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 15, 782–791 (2016).
Kang, S. K. et al. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–76 (2016).
Gao, Z. et al. Silicon nanowire arrays for label-free detection of DNA. Anal. Chem. 79, 3291–3297 (2007).
Patolsky, F., Zheng, G. & Lieber, C. M. Fabrication of silicon nanowire devices for ultrasensitive, label-free, real-time detection of biological and chemical species. Nat. Protoc. 1, 1701–1714 (2006).
Zheng, G., Patolsky, F., Cui, Y., Wang, W. U. & Lieber, C. M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 23, 1294–1301 (2005).
Park, C. W. et al. Thermally triggered degradation of transient electronic devices. Adv. Mater. 27, 3783–3788 (2015).
Troyk, P. R. Injectable electronic identification, monitoring, and stimulation systems. Annu. Rev. Biomed. Eng. 1, 176–209 (1999).
Johannessen, E. et al. Toward an injectable continuous osmotic glucose sensor. J. Diabetes Sci. Technol. 4, 882–892 (2010).
Liu, J. et al. Syringe-injectable electronics. Nat. Nanotechnol. 10, 629–636 (2015). This article presents the first example of electronics flexible enough to be injected through a syringe.
Xie, C. et al. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 14, 1286–1292 (2015).
Kim, T.-i. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Koh, A. et al. Ultrathin injectable sensors of temperature, thermal conductivity, and heat capacity for cardiac ablation monitoring. Adv. Healthcare Mater. 5, 373–381 (2016).
Luan, L. et al. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci. Adv. 3, e1591966 (2017).
Jiang, Y. et al. Heterogeneous silicon mesostructures for lipid-supported bioelectric interfaces. Nature Mater. 15, 1023–1030 (2016).
McCall, J. G. et al. Fabrication of flexible, multimodal light-emitting devices for wireless optogenetics. Nature Protoc. 8, 2413 (2013).
Hong, G. et al. Syringe injectable electronics: precise targeted delivery with quantitative input/output connectivity. Nano Lett. 15, 6979–6984 (2015).
Schuhmann, T. G., Yao, J., Hong, G., Fu, T.-M. & Lieber, C. M. Syringe-injectable electronics with a plug-and-play input/output interface. Nano Lett. 17, 5836–5842 (2017).
Zhou, T. et al. Syringe-injectable mesh electronics integrate seamlessly with minimal chronic immune response in the brain. Proc. Natl Acad. Sci. USA 114, 5894–5899 (2017).
Fu, T.-M. et al. Stable long-term chronic brain mapping at the single-neuron level. Nat. Methods 13, 875–882 (2016).
Duan, X. et al. Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor. Nat. Nanotechnol. 7, 173–178 (2012).
Tian, B. et al. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science 329, 830–834 (2010).
Landa, N. et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 117, 1388–1396 (2008).
Dvir, T., Timko, B. P., Kohane, D. S. & Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 6, 13–22 (2011).
Zsedenyi, A. et al. Gold nanoparticle-filled biodegradable photopolymer scaffolds induced muscle remodeling: in vitro and in vivo findings. Mater. Sci. Eng. C 72, 625–630 (2017).
Dvir, T. et al. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 6, 720–725 (2011).
Shevach, M., Maoz, B. M., Feiner, R., Shapira, A. & Dvir, T. Nanoengineering gold particle composite fibers for cardiac tissue engineering. J. Mater. Chem. B 1, 5210–5217 (2013).
Fleischer, S., Shevach, M., Feiner, R. & Dvir, T. Coiled fiber scaffolds embedded with gold nanoparticles improve the performance of engineered cardiac tissues. Nanoscale 6, 9410–9414 (2014).
Shevach, M., Fleischer, S., Shapira, A. & Dvir, T. Gold nanoparticle-decellularized matrix hybrids for cardiac tissue engineering. Nano Lett. 14, 5792–5796 (2014).
Baranes, K., Shevach, M., Shefi, O. & Dvir, T. Gold nanoparticle-decorated scaffolds promote neuronal differentiation and maturation. Nano Lett. 16, 2916–2920 (2015).
Radisic, M. et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc. Natl Acad. Sci. USA 101, 18029–18034 (2004).
Abbott, J. et al. CMOS nanoelectrode array for all-electrical intracellular electrophysiological imaging. Nat. Nanotechnol. 12, 460–466 (2017).
Lind, J. U. et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 16, 303–308 (2016).
Cho, S. & Yoon, J.-Y. Organ-on-a-chip for assessing environmental toxicants. Curr. Opin. Biotechnol. 45, 34–42 (2017).
Ribas, J. et al. Cardiovascular organ-on-a-chip platforms for drug discovery and development. Appl. In Vitro Toxicol. 2, 82–96 (2016).
Huh, D. et al. Reconstituting organ-level lung functions on a chip. Science 328, 1652–1658 (2010).
Wang, Z., Samanipour, R., Koo, K.-i. & Kim, K. Organ-on-a-chip platforms for drug delivery and cell characterization: a review. Sensors Mater. 27, 487–506 (2015).
Zheng, F. et al. Organ-on-a-chip systems: microengineering to biomimic living systems. Small 12, 2253–2282 (2016).
Tian, B. et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 11, 986–994 (2012). This is the first example of the integration of engineered tissues with electronics for sensing tissue function.
Feiner, R. et al. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat. Mater. 15, 679–685 (2016). This article describes the first example of the integration of electronics with engineered tissues for regulating their function by sensing, stimulating and releasing drugs.
Dai, X., Zhou, W., Gao, T., Liu, J. & Lieber, C. M. Three-dimensional mapping and regulation of action potential propagation in nanoelectronics-innervated tissues. Nat. Nanotechnol. 11, 776–782 (2016).
Yan, Z. et al. Mechanical assembly of complex, 3D mesostructures from releasable multilayers of advanced materials. Sci. Adv. 2, e1591014 (2016).
Lazarus, A. Remote, wireless, ambulatory monitoring of implantable pacemakers, cardioverter defibrillators, and cardiac resynchronization therapy systems: analysis of a worldwide database. Pacing Clin. Electrophysiol. 30, S2–S12 (2007).
Luo, Z. et al. Atomic gold–enabled three-dimensional lithography for silicon mesostructures. Science 348, 1451–1455 (2015).
Zimmerman, J. F. et al. Cellular uptake and dynamics of unlabeled freestanding silicon nanowires. Sci. Adv. 2, e1591039 (2016).
Dagdeviren, C. et al. Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proc. Natl Acad. Sci. USA 111, 1927–1932 (2014). This article presents energy harvesting from internal organs through piezoelectric components on a flexible device.
Deisseroth, K. Optogenetics. Nat. Methods 8, 26–29 (2011).
Wang, Y. et al. Wearable and highly sensitive graphene strain sensors for human motion monitoring. Adv. Funct. Mater. 24, 4666–4670 (2014).
Dagdeviren, C. et al. Flexible piezoelectric devices for gastrointestinal motility sensing. Nat. Biomed. Eng. 1, 807–817 (2017).
Merrill, D. R., Bikson, M. & Jefferys, J. G. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J. Neurosci. Methods 141, 170–198 (2005).
Cogan, S. F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 10, 275–309 (2008).
Zhao, Y. et al. Patch clamp technique: review of the current state of the art and potential contributions from nanoengineering. Proc. Inst. Mechan. Eng. Part N J. Nanomater Nanoeng. Nanosyst. 222, 1–11 (2008).
Spira, M. E. & Hai, A. Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 8, 83–94 (2013).
Shmoel, N. et al. Multisite electrophysiological recordings by self-assembled loose-patch-like junctions between cultured hippocampal neurons and mushroom-shaped microelectrodes. Sci. Rep. 6, 27110 (2016).
Hai, A., Shappir, J. & Spira, M. E. In-cell recordings by extracellular microelectrodes. Nat. Methods 7, 200–202 (2010).
Andreev, A., Gersuni, G. & Volokhov, A. On the electrical excitability of the human ear: on the effect of alternating currents on the affected auditory apparatus. J. Physiol. USSR 18, 250–265 (1935).
Djourno, A. & Eyries, C. Auditory prosthesis by means of a distant electrical stimulation of the sensory nerve with the use of an indwelt coiling. La Presse Méd. 65, 1417–1417 (1957).
Stokes, K. B. Drug dispensing body implantable lead. US Patent 4506680 (1985).
Benabid, A.-L., Pollak, P., Louveau, A., Henry, S. & De Rougemont, J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Stereotact. Funct. Neurosurgery 50, 344–346 (1987).
Gelbart, D. & Lichtenstein, S. V. Self-powered leadless pacemaker. US Patent 20070276444 A1 (2006).
Gozen, B. A., Tabatabai, A. O., Burak Ozdoganlar, O. B. & Majidi, C. High-density soft-matter electronics with micron-scale line width. Adv. Mater. 26, 5211–5216 (2014).
Nguyen, J. K. et al. Mechanically-compliant intracortical implants reduce the neuroinflammatory response. J. Neural Eng. 11, 056014 (2014).
Cobbe, S. & Poole-Wilson, P. The time of onset and severity of acidosis in myocardial ischaemia. J. Mol. Cell. Cardiol. 12, 745–760 (1980).
Van Der Vliet, A. & Bast, A. Role of reactive oxygen species in intestinal diseases. Free Radic. Biol. Med. 12, 499–513 (1992).
Kusters, J. G., van Vliet, A. H. & Kuipers, E. J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 19, 449–490 (2006).
Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 59, 1599–1613 (1992).
Yeh, W.-C. et al. Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med. Biol. 28, 467–474 (2002).
Wada, T. et al. Correlation of ultrasound-measured common carotid artery stiffness with pathological findings. Arterioscler. Thromb. 14, 479–482 (1994).
Tilleman, T. R., Neumann, M. H. & Tilleman, M. M. Analyses of skin waste during excision of benign skin lesions: is the surgical ellipse cut necessary? Plast. Reconstructive Surg. 119, 2343–2345 (2007).
Vey, E. et al. Degradation mechanism of poly (lactic-co-glycolic) acid block copolymer cast films in phosphate buffer solution. Polymer Degrad. Stabil. 93, 1859–1876 (2008).
Li, X., Kanjwal, M. A., Lin, L. & Chronakis, I. S. Electrospun polyvinyl-alcohol nanofibers as oral fast-dissolving delivery system of caffeine and riboflavin. Colloids Surf. B 103, 181–188 (2013).
Acknowledgements
R.F. thanks the Clore Scholarship programme, Marian Gertner Institute for Medical Nanosystems Fellowship and the Argentinian friends of Tel Aviv University. T.D. acknowledges support from the European Research Council (ERC) Starting Grant 637943, the Slezak Foundation and the Israeli Science Foundation (700/13).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Feiner, R., Dvir, T. Tissue–electronics interfaces: from implantable devices to engineered tissues. Nat Rev Mater 3, 17076 (2018). https://doi.org/10.1038/natrevmats.2017.76
Published:
DOI: https://doi.org/10.1038/natrevmats.2017.76
This article is cited by
-
Fully bioresorbable hybrid opto-electronic neural implant system for simultaneous electrophysiological recording and optogenetic stimulation
Nature Communications (2024)
-
Advances in Wireless, Batteryless, Implantable Electronics for Real-Time, Continuous Physiological Monitoring
Nano-Micro Letters (2024)
-
A highly adsorptive electrochemical fiber sensor for real-time and accurate detection of intracranial nitric oxide
Science China Materials (2024)
-
Laminin-coated electronic scaffolds with vascular topography for tracking and promoting the migration of brain cells after injury
Nature Biomedical Engineering (2023)
-
Highly conductive tissue-like hydrogel interface through template-directed assembly
Nature Communications (2023)