Abstract
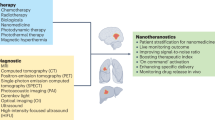
Advances in nanoparticle synthesis and engineering have produced nanoscale agents affording both therapeutic and diagnostic functions that are often referred to by the portmanteau ‘nanotheranostics’. The field is associated with many applications in the clinic, especially in cancer management. These include patient stratification, drug-release monitoring, imaging-guided focal therapy and post-treatment response monitoring. Recent advances in nanotheranostics have expanded this notion and enabled the characterization of individual tumours, the prediction of nanoparticle–tumour interactions, and the creation of tailor-designed nanomedicines for individualized treatment. Some of these applications require breaking the dogma that a nanotheranostic must combine both therapeutic and diagnostic agents within a single, physical entity; instead, it can be a general approach in which diagnosis and therapy are interwoven to solve clinical issues and improve treatment outcomes. In this Review, we describe the evolution and state of the art of cancer nanotheranostics, with an emphasis on clinical impact and translation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016). This perspective stimulates an interesting discussion on the efficiency of nanoparticle delivery to tumours.
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Kobayashi, H., Watanabe, R. & Choyke, P. L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 4, 81–89 (2013).
Prabhakar, U. et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 73, 2412–2417 (2013).
Bertrand, N., Wu, J., Xu, X., Kamaly, N. & Farokhzad, O. C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 66, 2–25 (2014).
Rajora, A. K., Ravishankar, D., Osborn, H. M. I. & Greco, F. Impact of the enhanced permeability and retention (EPR) effect and cathepsins levels on the activity of polymer–drug conjugates. Polymers 6, 2186–2220 (2014).
Shi, J., Kantoff, P. W., Wooster, R. & Farokhzad, O. C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17, 20–37 (2017). A noteworthy and comprehensive review on cancer nanomedicine development.
Kim, T. H., Lee, S. & Chen, X. Nanotheranostics for personalized medicine. Expert Rev. Mol. Diagn. 13, 257–269 (2013).
Mura, S. & Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 64, 1394–1416 (2012).
Kunjachan, S., Ehling, J., Storm, G., Kiessling, F. & Lammers, T. Noninvasive imaging of nanomedicines and nanotheranostics: principles, progress, and prospects. Chem. Rev. 115, 10907–10937 (2015).
Melancon, M. P., Stafford, R. J. & Li, C. Challenges to effective cancer nanotheranostics. J. Control. Release 164, 177–182 (2012).
Mura, S. & Couvreur, P. Nanotheranostics for Personalized Medicine (World Scientific, 2016).
Karathanasis, E. et al. Imaging nanoprobe for prediction of outcome of nanoparticle chemotherapy by using mammography. Radiology 250, 398–406 (2009).
Hansen, A. E. et al. Positron emission tomography based elucidation of the enhanced permeability and retention effect in dogs with cancer using copper-64 liposomes. ACS Nano 9, 6985–6995 (2015).
Harrington, K. J. et al. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin. Cancer Res. 7, 243–254 (2001).
Seymour, L. W. et al. Phase II studies of polymer–doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal cancer. Int. J. Oncol. 34, 1629–1636 (2009). A clinical study showing that a radiolabelled polymer–drug formulation, which is essentially a nanotheranostic, can be used to predict patient responses to nanoparticle therapy.
Arrieta, O. et al. First-line chemotherapy with liposomal doxorubicin plus cisplatin for patients with advanced malignant pleural mesothelioma: phase II trial. Br. J. Cancer 106, 1027–1032 (2012).
Arrieta, O. et al. High liposomal doxorubicin tumour tissue distribution, as determined by radiopharmaceutical labelling with (99m)Tc-LD, is associated with the response and survival of patients with unresectable pleural mesothelioma treated with a combination of liposomal doxorubicin and cisplatin. Cancer Chemother. Pharmacol. 74, 211–215 (2014).
Hamburg, M. A. & Collins, F. S. The path to personalized medicine. N. Engl. J. Med. 363, 301–304 (2010).
Weissleder, R., Schwaiger, M. C., Gambhir, S. S. & Hricak, H. Imaging approaches to optimize molecular therapies. Sci. Transl. Med. 8, 355ps16 (2016).
Miller, M. A. et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl. Med. 7, 314ra183 (2015). This study demonstrates the use of a clinically used nanoparticle formulation as a probe to accurately predict tumour accumulation and treatment efficacy of a range of nanotherapeutics.
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01770353 (2017).
Pérez-Medina, C. et al. Nanoreporter PET predicts the efficacy of anti-cancer nanotherapy. Nat. Commun. 7, 11838 (2016).
Miao, L. & Huang, L. Exploring the tumor microenvironment with nanoparticles. Cancer Treat. Res. 166, 193–226 (2015).
Jain, R. K. & Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653–664 (2010).
Barua, S. & Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today 9, 223–243 (2014).
Minchinton, A. I. & Tannock, I. F. Drug penetration in solid tumours. Nat. Rev. Cancer 6, 583–592 (2006).
Nakamura, Y., Mochida, A., Choyke, P. L. & Kobayashi, H. Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug. Chem. 27, 2225–2238 (2016).
Lee, J., Abdeen, A. A., Wycislo, K. L., Fan, T. M. & Kilian, K. A. Interfacial geometry dictates cancer cell tumorigenicity. Nat. Mater. 15, 856–862 (2016).
Manzoor, A. A. et al. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 72, 5566–5575 (2012).
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT00617981 (2017).
Smith, B. R. et al. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nat. Nanotechnol. 9, 481–487 (2014).
Matsumoto, Y. et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol. 11, 533–538 (2016).
Huang, J. et al. Magnetic nanoparticle facilitated drug delivery for cancer therapy with targeted and image-guided approaches. Adv. Funct. Mater. 26, 3818–3836 (2016).
Kaida, S. et al. Visible drug delivery by supramolecular nanocarriers directing to single-platformed diagnosis and therapy of pancreatic tumor model. Cancer Res. 70, 7031–7041 (2010).
Viglianti, B. L. et al. Chemodosimetry of in vivo tumor liposomal drug concentration using MRI. Magn. Reson. Med. 56, 1011–1018 (2006).
Ponce, A. M. et al. Magnetic resonance imaging of temperature-sensitive liposome release: drug dose painting and antitumor effects. J. Natl Cancer Inst. 99, 53–63 (2007).
Wang, D. et al. Novel dexamethasone–HPMA copolymer conjugate and its potential application in treatment of rheumatoid arthritis. Arthritis Res. Ther. 9, R2 (2007).
Kaittanis, C. et al. Environment-responsive nanophores for therapy and treatment monitoring via molecular MRI quenching. Nat. Commun. 5, 3384 (2014).
Zhao, Z. et al. Real-time monitoring of arsenic trioxide release and delivery by activatable T1 imaging. ACS Nano 9, 2749–2759 (2015).
Torchilin, V. P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 13, 813–827 (2014).
Mura, S., Nicolas, J. & Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12, 991–1003 (2013).
Gasselhuber, A. et al. Targeted drug delivery by high intensity focused ultrasound mediated hyperthermia combined with temperature-sensitive liposomes: computational modelling and preliminary in vivo validation. Int. J. Hyperthermia 28, 337–348 (2012).
Hsiao, Y. H., Kuo, S. J., Tsai, H. D., Chou, M. C. & Yeh, G. P. Clinical application of high-intensity focused ultrasound in cancer therapy. J. Cancer 7, 225–231 (2016).
You, Y. et al. Nanoparticle-enhanced synergistic HIFU ablation and transarterial chemoembolization for efficient cancer therapy. Nanoscale 8, 4324–4339 (2016).
Onuki, Y., Jacobs, I., Artemov, D. & Kato, Y. Noninvasive visualization of in vivo release and intratumoral distribution of surrogate MR contrast agent using the dual MR contrast technique. Biomaterials 31, 7132–7138 (2010).
Langereis, S. et al. A temperature-sensitive liposomal 1H CEST and 19F contrast agent for MR image-guided drug delivery. J. Am. Chem. Soc. 131, 1380–1381 (2009).
Zhu, X. et al. Long-circulating siRNA nanoparticles for validating Prohibitin1-targeted non-small cell lung cancer treatment. Proc. Natl Acad. Sci. USA 112, 7779–7784 (2015).
Sahin, U., Kariko, K. & Tureci, O. mRNA-based therapeutics — developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780 (2014).
Kormann, M. S. D. et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 29, 154–157 (2011).
Idris, N. M. et al. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med. 18, 1580–1585 (2012).
Lecaros, R. L., Huang, L., Lee, T. C. & Hsu, Y. C. Nanoparticle delivered VEGF-A siRNA enhances photodynamic therapy for head and neck cancer treatment. Mol. Ther. 24, 106–116 (2016).
Muhanna, N. et al. Phototheranostic porphyrin nanoparticles enable visualization and targeted treatment of head and neck cancer in clinically relevant models. Theranostics 5, 1428–1443 (2015).
Jin, C. S. et al. Nanoparticle-enabled selective destruction of prostate tumor using MRI-guided focal photothermal therapy. Prostate 76, 1169–1181 (2016).
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02680535 (2016).
Muhanna, N. et al. Multimodal image-guided surgical and photodynamic interventions in head and neck cancer: from primary tumor to metastatic drainage. Clin. Cancer Res. 22, 961–970 (2016).
Lin, J. et al. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano 7, 5320–5329 (2013).
Lin, J. et al. Multimodal-imaging-guided cancer phototherapy by versatile biomimetic theranostics with UV and γ-irradiation protection. Adv. Mater. 28, 3273–3279 (2016).
Lu, W. et al. Effects of photoacoustic imaging and photothermal ablation therapy mediated by targeted hollow gold nanospheres in an orthotopic mouse xenograft model of glioma. Cancer Res. 71, 6116–6121 (2011).
Kim, J. W., Galanzha, E. I., Shashkov, E. V., Moon, H. M. & Zharov, V. P. Golden carbon nanotubes as multimodal photoacoustic and photothermal high-contrast molecular agents. Nat. Nanotechnol. 4, 688–694 (2009).
Jaffray, D. A. Image-guided radiotherapy: from current concept to future perspectives. Nat. Rev. Clin. Oncol. 9, 688–699 (2012).
Strom, H. H. et al. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian lung cancer study group. Br. J. Cancer 109, 1467–1475 (2013).
Lukianova-Hleb, E. Y. et al. On-demand intracellular amplification of chemoradiation with cancer-specific plasmonic nanobubbles. Nat. Med. 20, 778–784 (2014).
Begg, A. C., Stewart, F. A. & Vens, C. Strategies to improve radiotherapy with targeted drugs. Nat. Rev. Cancer 11, 239–253 (2011).
Adams, S. R. et al. Anti-tubulin drugs conjugated to anti-ErbB antibodies selectively radiosensitize. Nat. Commun. 7, 13019 (2016).
Strom, T. J. et al. Increased acute mortality with chemoradiotherapy for locally advanced head and neck cancer in patients ≥70 years. J. Geriatr. Oncol. 8, 50–55 (2017).
Koukourakis, M. I. et al. Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non-small-cell lung cancer and head and neck cancer. J. Clin. Oncol. 17, 3512–3521 (1999).
Eblan, M. J. & Wang, A. Z. Improving chemoradiotherapy with nanoparticle therapeutics. Transl Cancer Res. 2, 320–329 (2013).
Werner, M. E. et al. Preclinical evaluation of Genexol-PM, a nanoparticle formulation of paclitaxel, as a novel radiosensitizer for the treatment of non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 86, 463–468 (2013).
Wang, E. C. et al. Nanoparticle formulations of histone deacetylase inhibitors for effective chemoradiotherapy in solid tumors. Biomaterials 51, 208–215 (2015).
Caster, J. M. et al. Nanoparticle delivery of chemosensitizers improve chemotherapy efficacy without incurring additional toxicity. Nanoscale 7, 2805–2811 (2015).
Karve, S. et al. Revival of the abandoned therapeutic wortmannin by nanoparticle drug delivery. Proc. Natl Acad. Sci. USA 109, 8230–8235 (2012).
Baumann, M. et al. Radiation oncology in the era of precision medicine. Nat. Rev. Cancer 16, 234–249 (2016).
Kunjachan, S. et al. Nanoparticle mediated tumor vascular disruption: a novel strategy in radiation therapy. Nano Lett. 15, 7488–7496 (2015).
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02379845?term (2017).
Bonvalot, S. et al. First-in-human study testing a new radioenhancer using nanoparticles (NBTXR3) activated by radiation therapy in patients with locally advanced soft tissue sarcomas. Clin. Cancer Res. 23, 908–917 (2017). A first-in-human study which uses hafnium oxide nanoparticles (NBTXR3) as radiosensitizers to aid cancer therapy.
Kotb, S. et al. Gadolinium-based nanoparticles and radiation therapy for multiple brain melanoma metastases: proof of concept before phase I trial. Theranostics 6, 418–427 (2016).
Detappe, A. et al. Advanced multimodal nanoparticles delay tumor progression with clinical radiation therapy. J. Control. Release 238, 103–113 (2016).
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02820454 (2016).
Chen, W. & Zhang, J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J. Nanosci. Nanotechnol. 6, 1159–1166 (2006).
Pogue, B. W. et al. Photodynamic therapy with verteporfin in the radiation-induced fibrosarcoma-1 tumor causes enhanced radiation sensitivity. Cancer Res. 63, 1025–1033 (2003).
Bulin, A. L. et al. X-Ray-induced singlet oxygen activation with nanoscintillator-coupled porphyrins. J. Phys. Chem. C 117, 21583–21589 (2013).
Tang, Y. G., Hu, J., Elmenoufy, A. H. & Yang, X. L. Highly efficient FRET system capable of deep photodynamic therapy established on X-ray excited mesoporous LaF3:Tb scintillating nanoparticles. ACS Appl. Mater. Interfaces 7, 12261–12269 (2015).
Chen, H. et al. Nanoscintillator-mediated X-ray inducible photodynamic therapy for in vivo cancer treatment. Nano Lett. 15, 2249–2256 (2015).
Wang, G. D. et al. X-Ray induced photodynamic therapy: a combination of radiotherapy and photodynamic therapy. Theranostics 6, 2295–2305 (2016).
Kotagiri, N., Sudlow, G. P., Akers, W. J. & Achilefu, S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nanotechnol. 10, 370–379 (2015).
Lukianova-Hleb, E. Y. et al. Intraoperative diagnostics and elimination of residual microtumours with plasmonic nanobubbles. Nat. Nanotechnol. 11, 525–532 (2016).
Kircher, M. F. et al. A brain tumor molecular imaging strategy using a new triple-modality MRI–photoacoustic–Raman nanoparticle. Nat. Med. 18, 829–834 (2012).
Phillips, E. et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 6, 260ra149 (2014).
Kim, S. E. et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat. Nanotechnol. 11, 977–985 (2016).
Brindle, K. New approaches for imaging tumour responses to treatment. Nat. Rev. Cancer 8, 94–107 (2008).
Shuhendler, A. J. et al. Molecular magnetic resonance imaging of tumor response to therapy. Sci. Rep. 5, 14759 (2015).
Bussink, J., Kaanders, J. H., van der Graaf, W. T. & Oyen, W. J. PET–CT for radiotherapy treatment planning and response monitoring in solid tumors. Nat. Rev. Clin. Oncol. 8, 233–242 (2011).
Jorgensen, J. T. et al. Single particle and PET-based platform for identifying optimal plasmonic mano-heaters for photothermal cancer therapy. Sci. Rep. 6, 30076 (2016).
Wang, Y. et al. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat. Mater. 13, 204–212 (2014).
Mi, P. et al. A pH-activatable nanoparticle with signal-amplification capabilities for non-invasive imaging of tumour malignancy. Nat. Nanotechnol. 11, 724–730 (2016).
Lee, S., Xie, J. & Chen, X. Activatable molecular probes for cancer imaging. Curr. Top. Med. Chem. 10, 1135–1144 (2010).
Guo, J. et al. 18F-alfatide ii and 18F-FDG dual-tracer dynamic PET for parametric, early prediction of tumor response to therapy. J. Nucl. Med. 55, 154–160 (2014).
Sun, X. et al. 18F-FPPRGD2 and 18F-FDG PET of response to abraxane therapy. J. Nucl. Med. 52, 140–146 (2011).
Notohamiprodjo, M. et al. Combined diffusion-weighted, blood oxygen level-dependent, and dynamic contrast-enhanced MRI for characterization and differentiation of renal cell carcinoma. Acad. Radiol. 20, 685–693 (2013).
Mittra, E. S. et al. Pilot pharmacokinetic and dosimetric studies of 18F-FPPRGD2: a PET radiopharmaceutical agent for imaging αvβ3 integrin levels. Radiology 260, 182–191 (2011).
Park, S. Y. et al. Assessment of early renal allograft dysfunction with blood oxygenation level-dependent MRI and diffusion-weighted imaging. Eur. J. Radiol. 83, 2114–2121 (2014).
Bedard, P. L., Hansen, A. R., Ratain, M. J. & Siu, L. L. Tumour heterogeneity in the clinic. Nature 501, 355–364 (2013).
Alizadeh, A. A. et al. Toward understanding and exploiting tumor heterogeneity. Nat. Med. 21, 846–853 (2015).
Junttila, M. R. & de Sauvage, F. J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501, 346–354 (2013).
Taurin, S., Nehoff, H. & Greish, K. Anticancer nanomedicine and tumor vascular permeability; where is the missing link? J. Control. Release 164, 265–275 (2012).
Toy, R. et al. Multimodal in vivo imaging exposes the voyage of nanoparticles in tumor microcirculation. ACS Nano 7, 3118–3129 (2013). A good example of using multiple imaging methods to comprehensively characterize tumours and understand the impact of individual factors on nanoparticle–tumour interactions.
Ullrich, R. T. et al. In-vivo visualization of tumor microvessel density and response to anti-angiogenic treatment by high resolution MRI in mice. PLoS ONE 6, e19592 (2011).
Theek, B. et al. Characterizing EPR-mediated passive drug targeting using contrast-enhanced functional ultrasound imaging. J. Control. Release 182, 83–89 (2014).
Chen, B. et al. Tumor vascular permeabilization by vascular-targeting photosensitization: effects, mechanism, and therapeutic implications. Clin. Cancer Res. 12, 917–923 (2006).
Hamzah, J. et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature 453, 410–414 (2008).
Jain, R. Measurements of tumor vascular leakiness using DCE in brain tumors: clinical applications. NMR Biomed. 26, 1042–1049 (2013).
Goyen, M. Gadofosveset-enhanced magnetic resonance angiography. Vasc. Health Risk Manag. 4, 1–9 (2008).
Niu, G. et al. In vivo labeling of serum albumin for PET. J. Nucl. Med. 55, 1150–1156 (2014).
Zhang, J. et al. Clinical translation of an albumin-binding PET radiotracer 68Ga-NEB. J. Nucl. Med. 56, 1609–1614 (2015).
Zhang, W. et al. Potential applications of using 68Ga-Evans blue PET/CT in the evaluation of lymphatic disorder: preliminary observations. Clin. Nucl. Med. 41, 302–308 (2016).
Stapleton, S. et al. A mathematical model of the enhanced permeability and retention effect for liposome transport in solid tumors. PLoS ONE 8, e81157 (2013).
Wong, A. D., Ye, M., Ulmschneider, M. B. & Searson, P. C. Quantitative analysis of the enhanced permeation and retention (EPR) effect. PLoS ONE 10, e0123461 (2015).
Tang, L. et al. Investigating the optimal size of anticancer nanomedicine. Proc. Natl Acad. Sci. USA 111, 15344–15349 (2014).
Cabral, H. et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 6, 815–823 (2011). A systematic study that compared the accumulation and therapeutic efficacy of different-sized, long-circulating, drug-loaded polymeric micelles. The study was conducted in animals bearing tumours of either high or low permeability.
Salvati, A. et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 8, 137–143 (2013).
Liu, R., Jiang, W., Walkey, C. D., Chan, W. C. W. & Cohen, Y. Prediction of nanoparticles–cell association based on corona proteins and physicochemical properties. Nanoscale 7, 9664–9675 (2015).
Wu, W. et al. Tumor-targeted aggregation of pH-sensitive nanocarriers for enhanced retention and rapid intracellular drug release. Polymer Chem. 5, 5668–5679 (2014).
Zhang, D. et al. In situ formation of nanofibers from purpurin 18–peptide conjugates and the assembly induced retention effect in tumor sites. Adv. Mater. 27, 6125–6130 (2015).
Wong, C. et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl Acad. Sci. USA 108, 2426–2431 (2011).
Li, H. J. et al. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc. Natl Acad. Sci. USA 113, 4164–4169 (2016).
Xu, R. et al. An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat. Biotechnol. 34, 414–418 (2016).
Lee, C. G. et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 60, 5565–5570 (2000).
Tong, R. T. et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 64, 3731–3736 (2004).
Willett, C. G. et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 10, 145–147 (2004).
Heist, R. S. et al. Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. Proc. Natl Acad. Sci. USA 112, 1547–1552 (2015).
Pham, E. et al. Preclinical efficacy of bevacizumab with CRLX101, an investigational nanoparticle-drug conjugate, in treatment of metastatic triple-negative breast cancer. Cancer Res. 76, 4493–4503 (2016).
Nehoff, H., Parayath, N. N., Domanovitch, L., Taurin, S. & Greish, K. Nanomedicine for drug targeting: strategies beyond the enhanced permeability and retention effect. Int. J. Nanomed. 9, 2539–2555 (2014).
Snyder, J. W., Greco, W. R., Bellnier, D. A., Vaughan, L. & Henderson, B. W. Photodynamic therapy: a means to enhanced drug delivery to tumors. Cancer Res. 63, 8126–8131 (2003).
Zhen, Z. et al. Tumor vasculature targeted photodynamic therapy for enhanced delivery of nanoparticles. ACS Nano 8, 6004–6013 (2014).
Carpentier, A. et al. Clinical trial of blood–brain barrier disruption by pulsed ultrasound. Sci. Transl Med. 8, 343re2 (2016). A first-in-human trial showing initial successes on disrupting the blood–brain barrier with ultrasound.
Leinenga, G. & Gotz, J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer's disease mouse model. Sci. Transl. Med. 7, 278ra33 (2015).
Kobayashi, H. et al. Application of a macromolecular contrast agent for detection of alterations of tumor vessel permeability induced by radiation. Clin. Cancer Res. 10, 7712–7720 (2004).
Sano, K., Nakajima, T., Choyke, P. L. & Kobayashi, H. Markedly enhanced permeability and retention effects induced by photo-immunotherapy of tumors. ACS Nano 7, 717–724 (2013).
Suzuki, M., Hori, K., Abe, I., Saito, S. & Sato, H. A new approach to cancer chemotherapy: selective enhancement of tumor blood flow with angiotensin ii. J. Natl Cancer Inst. 67, 663–669 (1981).
Li, C. J., Miyamoto, Y., Kojima, Y. & Maeda, H. Augmentation of tumour delivery of macromolecular drugs with reduced bone marrow delivery by elevating blood pressure. Br. J. Cancer 67, 975–980 (1993).
Diop-Frimpong, B., Chauhan, V. P., Krane, S., Boucher, Y. & Jain, R. K. Losartan inhibits collagen i synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl Acad. Sci. USA 108, 2909–2914 (2011).
Buckway, B., Frazier, N., Gormley, A. J., Ray, A. & Ghandehari, H. Gold nanorod-mediated hyperthermia enhances the efficacy of HPMA copolymer–90Y conjugates in treatment of prostate tumors. Nucl. Med. Biol. 41, 282–289 (2014).
Tamarov, K. P. et al. Radio frequency radiation-induced hyperthermia using Si nanoparticle-based sensitizers for mild cancer therapy. Sci. Rep. 4, 7034 (2014).
Yin, D. et al. Convection-enhanced delivery improves distribution and efficacy of tumor-selective retroviral replicating vectors in a rodent brain tumor model. Cancer Gene Ther. 20, 336–341 (2013).
Perlstein, B. et al. Convection-enhanced delivery of maghemite nanoparticles: Increased efficacy and MRI monitoring. Neuro Oncol. 10, 153–161 (2008).
McNeil, S. E. Evaluation of nanomedicines: stick to the basics. Nat. Rev. Mater. 1, 16073 (2016).
Lammers, T. et al. Cancer nanomedicine: is targeting our target? Nat. Rev. Mater. 1, 16069 (2016).
Schork, N. J. Personalized medicine: time for one-person trials. Nature 520, 609–611 (2015).
Scuffham, P. A. et al. Using N-of-1 trials to improve patient management and save costs. J. Gen. Intern. Med. 25, 906–913 (2010).
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02116621 (2015).
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02142036 (2017).
Irvine, D. J. Materializing the future of vaccines and immunotherapy. Nat. Rev. Mater. 1, 15008 (2016).
Hubbell, J. A., Thomas, S. N. & Swartz, M. A. Materials engineering for immunomodulation. Nature 462, 449–460 (2009).
Stephan, M. T., Moon, J. J., Um, S. H., Bershteyn, A. & Irvine, D. J. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 16, 1035–1041 (2010).
Rosenberg, S. A. Raising the bar: the curative potential of human cancer immunotherapy. Sci. Transl Med. 4, 127ps8 (2012).
Hailemichael, Y. et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat. Med. 19, 465–472 (2013).
Moon, J. J., Huang, B. & Irvine, D. J. Engineering nano- and microparticles to tune immunity. Adv. Mater. 24, 3724–3746 (2012).
Irvine, D. J., Hanson, M. C., Rakhra, K. & Tokatlian, T. Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 115, 11109–11146 (2015).
Zhu, G. et al. DNA–inorganic hybrid nanovaccine for cancer immunotherapy. Nanoscale 8, 6684–6692 (2016).
Kuai, R., Ochyl, L. J., Bahjat, K. S., Schwendeman, A. & Moon, J. J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 16, 489–496 (2017).
Nguyen, D. N. et al. Lipid-derived nanoparticles for immunostimulatory RNA adjuvant delivery. Proc. Natl Acad. Sci. USA 109, E797–E803 (2012).
Jeanbart, L. & Swartz, M. A. Engineering opportunities in cancer immunotherapy. Proc. Natl Acad. Sci. USA 112, 14467–14472 (2015).
Shao, K. et al. Nanoparticle-based immunotherapy for cancer. ACS Nano 9, 16–30 (2015).
Radovic-Moreno, A. F. et al. Immunomodulatory spherical nucleic acids. Proc. Natl Acad. Sci. USA 112, 3892–3897 (2015).
Koshy, S. T., Cheung, A. S., Gu, L., Graveline, A. R. & Mooney, D. J. Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv. Biosystems 1, 1600013 (2017).
Hanson, M. C. et al. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J. Clin. Invest. 125, 2532–2546 (2015).
Liu, H. et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 507, 519–522 (2014).
Guo, Y. et al. Erythrocyte membrane-enveloped polymeric nanoparticles as nanovaccine for induction of antitumor immunity against melanoma. ACS Nano 9, 6918–6933 (2015).
Fang, R. H. et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 14, 2181–2188 (2014).
Cheung, A. S., Koshy, S. T., Stafford, A. G., Bastings, M. M. C. & Mooney, D. J. Adjuvant-loaded subcellular vesicles derived from disrupted cancer cells for cancer vaccination. Small 12, 2321–2333 (2016).
Barber, G. N. STING: infection, inflammation and cancer. Nat. Rev. Immunol. 15, 760–770 (2015).
Guermonprez, P. et al. ER–phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425, 397–402 (2003).
Burgdorf, S., Schölz, C., Kautz, A., Tampé, R. & Kurts, C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat. Immunol. 9, 558–566 (2008).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–420 (2013).
Schlake, T., Thess, A., Fotin-Mleczek, M. & Kallen, K.-J. Developing mRNA-vaccine technologies. RNA Biol. 9, 1319–1330 (2012).
Benteyn, D., Heirman, C., Bonehill, A., Thielemans, K. & Breckpot, K. mRNA-based dendritic cell vaccines. Expert Rev. Vaccines 14, 161–176 (2014).
Sahin, U., Karikó, K. & Türeci, Ö . mRNA-based therapeutics — developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780 (2014).
Li, J. et al. Structurally programmed assembly of translation initiation nanoplex for superior mRNA delivery. ACS Nano 11, 2531–2544 (2017).
Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016).
Kreiter, S. et al. Mutant MHC class ii epitopes drive therapeutic immune responses to cancer. Nature 520, 692–696 (2015).
Mitragotri, S. et al. Drug delivery research for the future: expanding the nano horizons and beyond. J. Control. Release 246, 183–184 (2017).
Acknowledgements
We thank H. S. Eden for critically reading the manuscript. This work was supported in part by the Intramural Research Program, National Institute of Biomedical Imaging and Bioengineering, US National Institutes of Health (NIH); and by the Department of Defense (CDMRP grant CA140666), National Science Foundation (CAREER grant NSF1552617), University of Georgia–Georgia Regents University (seed grant) and NIH (R01 grants R01EB022596 and R01NS093314).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Chen, H., Zhang, W., Zhu, G. et al. Rethinking cancer nanotheranostics. Nat Rev Mater 2, 17024 (2017). https://doi.org/10.1038/natrevmats.2017.24
Published:
DOI: https://doi.org/10.1038/natrevmats.2017.24
This article is cited by
-
Targeting ferroptosis for leukemia therapy: exploring novel strategies from its mechanisms and role in leukemia based on nanotechnology
European Journal of Medical Research (2024)
-
Ferroptosis: Emerging mechanisms, biological function, and therapeutic potential in cancer and inflammation
Cell Death Discovery (2024)
-
Aggregation-induced Emission Probe for Fluorescence/Photoacoustic Dual-modality Imaging and Photodynamic/Photothermal Treatment
Chemical Research in Chinese Universities (2024)
-
Nanotechnology in leukemia: diagnosis, efficient-targeted drug delivery, and clinical trials
European Journal of Medical Research (2023)
-
Biomimetic liposomal nanozymes improve breast cancer chemotherapy with enhanced penetration and alleviated hypoxia
Journal of Nanobiotechnology (2023)