Abstract
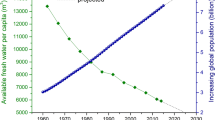
Membrane-based separations for water purification and desalination have been increasingly applied to address the global challenges of water scarcity and the pollution of aquatic environments. However, progress in water purification membranes has been constrained by the inherent limitations of conventional membrane materials. Recent advances in methods for controlling the structure and chemical functionality in polymer films can potentially lead to new classes of membranes for water purification. In this Review, we first discuss the state of the art of existing membrane technologies for water purification and desalination, highlight their inherent limitations and establish the urgent requirements for next-generation membranes. We then describe molecular-level design approaches towards fabricating highly selective membranes, focusing on novel materials such as aquaporin, synthetic nanochannels, graphene and self-assembled block copolymers and small molecules. Finally, we highlight promising membrane surface modification approaches that minimize interfacial interactions and enhance fouling resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Elimelech, M. The global challenge for adequate and safe water. J. Water Supply Res. Technol. 55, 3–10 (2006).
Shannon, M. A. et al. Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008).
Schwarzenbach, R. P. et al. The challenge of micropollutants in aquatic systems. Science 313, 1072–1077 (2006).
Kolpin, D. W. et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environ. Sci. Technol. 36, 1202–1211 (2002).
Tang, J. Y., Busetti, F., Charrois, J. W. & Escher, B. I. Which chemicals drive biological effects in wastewater and recycled water? Water Res. 60, 289–299 (2014).
Vidic, R. D., Brantley, S. L., Vandenbossche, J. M., Yoxtheimer, D. & Abad, J. D. Impact of shale gas development on regional water quality. Science 340, 1235009 (2013).
Shaffer, D. L. et al. Desalination and reuse of high-salinity shale gas produced water: drivers, technologies, and future directions. Environ. Sci. Technol. 47, 9569–9583 (2013).
Gregory, K. B., Vidic, R. D. & Dzombak, D. A. Water management challenges associated with the production of shale gas by hydraulic fracturing. Elements 7, 181–186 (2011).
Bond, R. & Veerapaneni, S. Zero liquid discharge for inland desalination (Awwa Research Foundation, 2007).
Mickley, M. Survey of high-recovery and zero liquid discharge technologies for water utilities (WateReuse Foundation, 2008).
van Loosdrecht, M. C. M. & Brdjanovic, D. Anticipating the next century of wastewater treatment. Science 344, 1452–1453 (2014).
Grant, S. B. et al. Taking the “waste” out of “wastewater” for human water security and ecosystem sustainability. Science 337, 681–686 (2012).
Sales, C. M. & Lee, P. K. Resource recovery from wastewater: application of meta-omics to phosphorus and carbon management. Curr. Opin. Biotechnol. 33, 260–267 (2015).
Wang, X. et al. Probabilistic evaluation of integrating resource recovery into wastewater treatment to improve environmental sustainability. Proc. Natl Acad. Sci. USA 112, 1630–1635 (2015).
Elimelech, M. & Phillip, W. A. The future of seawater desalination: energy, technology, and the environment. Science 333, 712–717 (2011).
Semiat, R. Energy issues in desalination processes. Environ. Sci. Technol. 42, 8193–8201 (2008).
Baker, R. W. Membrane Technology and Applications (John Wiley & Sons, 2012).
Gin, D. L. & Noble, R. D. Designing the next generation of chemical separation membranes. Science 332, 674–676 (2011).
Tang, C. Y., Kwon, Y.-N. & Leckie, J. O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes. Desalination 242, 168–182 (2009).
Lu, X. et al. Elements provide a clue: nanoscale characterization of thin-film composite polyamide membranes. ACS Appl. Mater. Interfaces 7, 16917–16922 (2015).
Karan, S., Jiang, Z. & Livingston, A. G. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 348, 1347–1351 (2015).
Kwak, S. Y., Jung, S. G. & Kim, S. H. Structure-motion-performance relationship of flux-enhanced reverse osmosis (RO) membranes composed of aromatic polyamide thin films. Environ. Sci. Technol. 35, 4334–4340 (2001).
Mehta, A. & Zydney, A. L. Permeability and selectivity analysis for ultrafiltration membranes. J. Membr. Sci. 249, 245–249 (2005).
Geise, G. M., Paul, D. R. & Freeman, B. D. Fundamental water and salt transport properties of polymeric materials. Prog. Polym. Sci. 39, 1–42 (2014).
Geise, G. M., Park, H. B., Sagle, A. C., Freeman, B. D. & McGrath, J. E. Water permeability and water/salt selectivity tradeoff in polymers for desalination. J. Membr. Sci. 369, 130–138 (2011). A water–salt selectivity trade-off for desalination using polymeric membranes is proposed for the first time in this study.
Robeson, L. M. The upper bound revisited. J. Membr. Sci. 320, 390–400 (2008).
Yip, N. Y. & Elimelech, M. Performance limiting effects in power generation from salinity gradients by pressure retarded osmosis. Environ. Sci. Technol. 45, 10273–10282 (2011).
Iwahashi, H. et al. Advanced RO system for high temperature and high concentration seawater desalination at the Arabian Gulf. IDA World Congress (San Diego, 2015).
Cohen-Tanugi, D., McGovern, R. K., Dave, S. H., Lienhard, J. H. & Grossman, J. C. Quantifying the potential of ultra-permeable membranes for water desalination. Energy Environ. Sci. 7, 1134–1141 (2014).
Deshmukh, A., Yip, N. Y., Lin, S. & Elimelech, M. Desalination by forward osmosis: identifying performance limiting parameters through module-scale modeling. J. Membr. Sci. 491, 159–167 (2015).
Singh, R. Production of high-purity water by membrane processes. Desalin. Water Treat. 3, 99–110 (2012).
Miyashita, Y., Park, S.-H., Hyung, H., Huang, C.-H. & Kim, J.-H. Removal of N-nitrosamines and their precursors by nanofiltration and reverse osmosis membranes. J. Environ. Eng. 135, 788–795 (2009).
Ozaki, H. & Li, H. Rejection of organic compounds by ultra-low pressure reverse osmosis membrane. Water Res. 36, 123–130 (2002).
Fritzmann, C., Lö wenberg, J., Wintgens, T. & Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 216, 1–76 (2007).
Le-Clech, P., Chen, V. & Fane, T. A. G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 284, 17–53 (2006).
Cheryan, M. Ultrafiltration and Microfiltration Handbook 31–70 (CRC Press, 1998).
Matthiasson, E. The role of macromolecular adsorption in fouling of ultrafiltration membranes. J. Membr. Sci. 16, 23–36 (1983).
Belfort, G., Davis, R. H. & Zydney, A. L. The behavior of suspensions and macromolecular solutions in crossflow microfiltration. J. Membr. Sci. 96, 1–58 (1994).
Elimelech, M., Zhu, X., Childress, A. E. & Hong, S. Role of membrane surface morphology in colloidal fouling of cellulose acetate and composite aromatic polyamide reverse osmosis membranes. J. Membr. Sci. 127, 101–109 (1997). The roughness of polyamide thin-film composite membrane surfaces is shown in this study to exacerbate fouling.
Herzberg, M. & Elimelech, M. Biofouling of reverse osmosis membranes: role of biofilm-enhanced osmotic pressure. J. Membr. Sci. 295, 11–20 (2007).
Li, Q. & Elimelech, M. Organic fouling and chemical cleaning of nanofiltration membranes: measurements and mechanisms. Environ. Sci. Technol. 38, 4683–4693 (2004).
Mi, B. & Elimelech, M. Gypsum scaling and cleaning in forward osmosis: measurements and mechanisms. Environ. Sci. Technol. 44, 2022–2028 (2010).
Borgnia, M. J., Kozono, D., Calamita, G., Maloney, P. C. & Agre, P. Functional reconstitution and characterization of AqpZ, the E. coli water channel protein. J. Mol. Biol. 291, 1169–1179 (1999).
Beitz, E., Wu, B., Holm, L. M., Schultz, J. E. & Zeuthen, T. Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc. Natl Acad. Sci. USA 103, 269–274 (2006).
Hub, J. S. & de Groot, B. L. Mechanism of selectivity in aquaporins and aquaglyceroporins. Proc. Natl Acad. Sci. USA 105, 1198–1203 (2008).
Sui, H., Han, B. G., Lee, J. K., Walian, P. & Jap, B. K. Structural basis of water-specific transport through the AQP1 water channel. Nature 414, 872–878 (2001). In this X-ray crystallography study, a high-resolution structure of aquaporin 1 was attained, which elucidated the basis for its selectivity for water.
Murata, K. et al. Structural determinants of water permeation through aquaporin-1. Nature 407, 599–605 (2000).
Savage, D. F., O'Connell, J. D., Miercke, L. J. W., Finer-Moore, J. & Stroud, R. M. Structural context shapes the aquaporin selectivity filter. Proc. Natl Acad. Sci. USA 107, 17164–17169 (2010).
Wang, M. et al. Layer-by-layer assembly of aquaporin Z-incorporated biomimetic membranes for water purification. Environ. Sci. Technol. 49, 3761–3768 (2015).
Wang, H. et al. Highly permeable and selective pore-spanning biomimetic membrane embedded with aquaporin Z. Small 8, 1185–1190 (2012).
Kumar, M., Grzelakowski, M., Zilles, J., Clark, M. & Meier, W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein aquaporin Z. Proc. Natl Acad. Sci. USA 104, 20719–20724 (2007).
Zhong, P. S., Chung, T.-S., Jeyaseelan, K. & Armugam, A. Aquaporin-embedded biomimetic membranes for nanofiltration. J. Membr. Sci. 407–408, 27–33 (2012).
Hummer, G., Rasaiah, J. C. & Noworyta, J. P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 414, 188–190 (2001). This study provides the first demonstration, using molecular dynamics, of ultra-fast water permeation through the interior of carbon nanotubes.
Corry, B. Designing carbon nanotube membranes for efficient water desalination. J. Phys. Chem. B 112, 1427–1434 (2008).
Majumder, M., Chopra, N., Andrews, R. & Hinds, B. J. Nanoscale hydrodynamics: enhanced flow in carbon nanotubes. Nature 438, 44 (2005).
Holt, J. K. et al. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 312, 1034–1037 (2006).
Hinds, B. J. et al. Aligned multiwalled carbon nanotube membranes. Science 303, 62–65 (2004).
Majumder, M., Chopra, N. & Hinds, B. J. Effect of tip functionalization on transport through vertically oriented carbon nanotube membranes. J. Am. Chem. Soc. 127, 9062–9070 (2005).
Fornasiero, F. et al. Ion exclusion by sub-2-nm carbon nanotube pores. Proc. Natl Acad. Sci. USA 105, 17250–17255 (2008).
Corry, B. Water and ion transport through functionalised carbon nanotubes: implications for desalination technology. Energy Environ. Sci. 4, 751–759 (2011).
Chan, W. F. et al. Zwitterion functionalized carbon nanotube/polyamide nanocomposite membranes for water desalination. ACS Nano 7, 5308–5319 (2013).
Sanchez-Valencia, J. R. et al. Controlled synthesis of single-chirality carbon nanotubes. Nature 512, 61–64 (2014).
Shen, Y. X. et al. Highly permeable artificial water channels that can self-assemble into two-dimensional arrays. Proc. Natl Acad. Sci. USA 112, 9810–9815 (2015).
Hu, X. B., Chen, Z., Tang, G., Hou, J. L. & Li, Z. T. Single-molecular artificial transmembrane water channels. J. Am. Chem. Soc. 134, 8384–8387 (2012).
Hourani, R. et al. Processable cyclic peptide nanotubes with tunable interiors. J. Am. Chem. Soc. 133, 15296–15299 (2011).
Zhou, X. et al. Self-assembling subnanometer pores with unusual mass-transport properties. Nat. Commun. 3, 949 (2012). Highly selective artificial water channels that operate by rigid molecular sieving are reported in this study.
Mauter, M. S., Elimelech, M. & Osuji, C. O. Nanocomposites of vertically aligned single-walled carbon nanotubes by magnetic alignment and polymerization of a lyotropic precursor. ACS Nano 4, 6651–6658 (2010).
Xu, T. et al. Subnanometer porous thin films by the co-assembly of nanotube subunits and block copolymers. ACS Nano 5, 1376–1384 (2011).
Gin, D. L., Gu, W., Pindzola, B. A. & Zhou, W. J. Polymerized lyotropic liquid crystal assemblies for materials applications. Acc. Chem. Res. 34, 973–980 (2001).
Gin, D. L., Bara, J. E., Noble, R. D. & Elliott, B. J. Polymerized lyotropic liquid crystal assemblies for membrane applications. Macromol. Rapid. Commun. 29, 367–389 (2008).
Zhang, Y., Sargent, J. L., Boudouris, B. W. & Phillip, W. A. Nanoporous membranes generated from self-assembled block polymer precursors: quo vadis? J. Appl. Polym. Sci. 132, 41683 (2015).
Jackson, E. A. & Hillmyer, M. A. Nanoporous membranes derived from block copolymers: from drug delivery to water filtration. ACS Nano 4, 3548–3553 (2010).
Zalusky, A. S., Olayo-Valles, R., Wolf, J. H. & Hillmyer, M. A. Ordered nanoporous polymers from polystyrene–polylactide block copolymers. J. Am. Chem. Soc. 124, 12761–12773 (2002).
Sorenson, G. P., Coppage, K. L. & Mahanthappa, M. K. Unusually stable aqueous lyotropic gyroid phases from gemini dicarboxylate surfactants. J. Am. Chem. Soc. 133, 14928–14931 (2011).
Hatakeyama, E. S., Wiesenauer, B. R., Gabriel, C. J., Noble, R. D. & Gin, D. L. Nanoporous, bicontinuous cubic lyotropic liquid crystal networks via polymerizable gemini ammonium surfactants. Chem. Mater. 22, 4525–4527 (2010).
Zhou, M. et al. New type of membrane material for water desalination based on a cross-linked bicontinuous cubic lyotropic liquid crystal assembly. J. Am. Chem. Soc. 129, 9574–9575 (2007).
Pindzola, B. A., Jin, J. & Gin, D. L. Cross-linked normal hexagonal and bicontinuous cubic assemblies via polymerizable gemini amphiphiles. J. Am. Chem. Soc. 125, 2940–2949 (2003).
Soberats, B. et al. 3D Anhydrous proton-transporting nanochannels formed by self-assembly of liquid crystals composed of a sulfobetaine and a sulfonic acid. J. Am. Chem. Soc. 135, 15286–15289 (2013).
Kerr, R. L., Miller, S. A., Shoemaker, R. K., Elliott, B. J. & Gin, D. L. New type of Li ion conductor with 3D interconnected nanopores via polymerization of a liquid organic electrolyte-filled lyotropic liquid-crystal assembly. J. Am. Chem. Soc. 131, 15972–15973 (2009).
Smith, R. C., Fischer, W. M. & Gin, D. L. Ordered poly(p-phenylenevinylene) matrix nanocomposites via lyotropic liquid-crystalline monomers. J. Am. Chem. Soc. 119, 4092–4093 (1997). This is an early demonstration of the formation of a nanoporous polymeric structure using surfactants based on polymerizable gallic acid.
Zhou, M., Kidd, T. J., Noble, R. D. & Gin, D. L. Supported lyotropic liquid-crystal polymer membranes: promising materials for molecular-size-selective aqueous nanofiltration. Adv. Mater. 17, 1850–1853 (2005).
Broer, D. J., Bastiaansen, C. M., Debije, M. G. & Schenning, A. P. Functional organic materials based on polymerized liquid-crystal monomers: supramolecular hydrogen-bonded systems. Angew. Chem. Int. Ed. Engl. 51, 7102–7109 (2012).
Henmi, M. et al. Self-organized liquid-crystalline nanostructured membranes for water treatment: selective permeation of ions. Adv. Mater. 24, 2238–2241 (2012).
Deng, H., Gin, D. L. & Smith, R. C. Polymerizable lyotropic liquid crystals containing transition-metal and lanthanide ions: architectural control and introduction of new properties into nanostructured polymers. J. Am. Chem. Soc. 120, 3522–3523 (1998).
Lee, H.-K. et al. Synthesis of a nanoporous polymer with hexagonal channels from supramolecular discotic liquid crystals. Angew. Chem. Int. Ed. Engl. 40, 2669–2671 (2001).
Ishida, Y. et al. Guest-responsive covalent frameworks by the cross-linking of liquid-crystalline salts: tuning of lattice flexibility by the design of polymerizable units. Chemistry 17, 14752–14762 (2011).
Feng, X. et al. Scalable fabrication of polymer membranes with vertically aligned 1 nm pores by magnetic field directed self-assembly. ACS Nano 8, 11977–11986 (2014).
Gopinadhan, M. et al. Thermally switchable aligned nanopores by magnetic-field directed self-assembly of block copolymers. Adv. Mater. 26, 5148–5154 (2014).
Feng, X. et al. Thin polymer films with continuous vertically aligned 1 nm pores fabricated by soft confinement. ACS Nano 10, 150–158 (2015).
Peinemann, K. V., Abetz, V. & Simon, P. F. Asymmetric superstructure formed in a block copolymer via phase separation. Nat. Mater. 6, 992–996 (2007). In this study, block copolymer self-assembly and phase inversion are combined for the first time to readily form porous membranes with a low pore size polydispersity.
Phillip, W. A. et al. Tuning structure and properties of graded triblock terpolymer-based mesoporous and hybrid films. Nano Lett. 11, 2892–2900 (2011).
Gu, Y. & Wiesner, U. Tailoring pore size of graded mesoporous block copolymer membranes: moving from ultrafiltration toward nanofiltration. Macromolecules 48, 6153–6159 (2015).
Clodt, J. I. et al. Performance study of isoporous membranes with tailored pore sizes. J. Membr. Sci. 495, 334–340 (2015).
Seo, M. & Hillmyer, M. A. Reticulated nanoporous polymers by controlled polymerization-induced microphase separation. Science 336, 1422–1425 (2012).
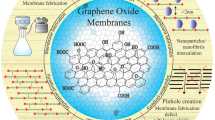
Joshi, R. K. et al. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 343, 752–754 (2014). This is an experimental demonstration of molecular sieving through graphene oxide laminates, albeit with a size cut-off that is too large for desalination.
Nair, R. R., Wu, H. A., Jayaram, P. N., Grigorieva, I. V. & Geim, A. K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 335, 442–444 (2012).
Surwade, S. P. et al. Water desalination using nanoporous single-layer graphene. Nat. Nanotechnol. 10, 459–464 (2015).
Russo, C. J. & Golovchenko, J. A. Atom-by-atom nucleation and growth of graphene nanopores. Proc. Natl Acad. Sci. USA 109, 5953–5957 (2012).
Cohen-Tanugi, D. & Grossman, J. C. Water desalination across nanoporous graphene. Nano Lett. 12, 3602–3608 (2012).
O'Hern, S. C. et al. Selective ionic transport through tunable subnanometer pores in single-layer graphene membranes. Nano Lett. 14, 1234–1241 (2014).
O'Hern, S. C. et al. Nanofiltration across defect-sealed nanoporous monolayer graphene. Nano Lett. 15, 3254–3260 (2015).
O'Hern, S. C. et al. Selective molecular transport through intrinsic defects in a single layer of CVD graphene. ACS Nano 6, 10130–10138 (2012).
Mi, B. Graphene oxide membranes for ionic and molecular sieving. Science 343, 740–742 (2014).
Yeh, C. N., Raidongia, K., Shao, J., Yang, Q. H. & Huang, J. On the origin of the stability of graphene oxide membranes in water. Nat. Chem. 7, 166–170 (2014).
Su, Y. et al. Impermeable barrier films and protective coatings based on reduced graphene oxide. Nat. Commun. 5, 4843 (2014).
Liu, H., Wang, H. & Zhang, X. Facile fabrication of freestanding ultrathin reduced graphene oxide membranes for water purification. Adv. Mater. 27, 249–254 (2015).
Hung, W.-S. et al. Cross-linking with diamine monomers to prepare composite graphene oxide-framework membranes with varying d-spacing. Chem. Mater. 26, 2983–2990 (2014).
Zhang, Y., Zhang, S. & Chung, T. S. Nanometric graphene oxide framework membranes with enhanced heavy metal removal via nanofiltration. Environ. Sci. Technol. 49, 10235–10242 (2015).
Ostuni, E., Chapman, R. G., Holmlin, R. E., Takayama, S. & Whitesides, G. M. A survey of structure–property relationships of surfaces that resist the adsorption of protein. Langmuir 17, 5605–5620 (2001). Through adsorption measurements of proteins on model surfaces with defined functional groups, this study describes general characteristics of non-fouling surfaces.
Banerjee, I., Pangule, R. C. & Kane, R. S. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 23, 690–718 (2011).
Jiang, S. & Cao, Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 22, 920–932 (2010).
Callow, J. A. & Callow, M. E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2, 244 (2011).
Kang, G. D. & Cao, Y. M. Development of antifouling reverse osmosis membranes for water treatment: a review. Water Res. 46, 584–600 (2012).
Rana, D. & Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 110, 2448–2471 (2010).
Shaffer, D. L., Jaramillo, H., Romero-Vargas Castrillón, S., Lu, X. & Elimelech, M. Post-fabrication modification of forward osmosis membranes with a poly(ethylene glycol) block copolymer for improved organic fouling resistance. J. Membr. Sci. 490, 209–219 (2015).
Van Wagner, E. M., Sagle, A. C., Sharma, M. M., La, Y.-H. & Freeman, B. D. Surface modification of commercial polyamide desalination membranes using poly(ethylene glycol) diglycidyl ether to enhance membrane fouling resistance. J. Membr. Sci. 367, 273–287 (2011).
Barbey, R. et al. Polymer brushes via surface-initiated controlled radical polymerization: synthesis, characterization, properties, and applications. Chem. Rev. 109, 5437–5527 (2009).
Ye, G., Lee, J., Perreault, F. & Elimelech, M. Controlled architecture of dual-functional block copolymer brushes on thin-film composite membranes for integrated “defending” and “attacking” strategies against biofouling. ACS Appl. Mater. Interfaces 7, 23069–23079 (2015).
Kang, S., Asatekin, A., Mayes, A. M. & Elimelech, M. Protein antifouling mechanisms of PAN UF membranes incorporating PAN-g-PEO additive. J. Membr. Sci. 296, 42–50 (2007).
Asatekin, A., Kang, S., Elimelech, M. & Mayes, A. M. Anti-fouling ultrafiltration membranes containing polyacrylonitrile-graft-poly(ethylene oxide) comb copolymer additives. J. Membr. Sci. 298, 136–146 (2007). The in situ surface segregation approach is used in this study to readily fabricate fouling-resistant polyacrylonitrile UF membranes.
Chen, W. et al. Engineering a robust, versatile amphiphilic membrane surface through forced surface segregation for ultralow flux-decline. Adv. Funct. Mater. 21, 191–198 (2011).
Chen, W. et al. Efficient wastewater treatment by membranes through constructing tunable antifouling membrane surfaces. Environ. Sci. Technol. 45, 6545–6552 (2011).
Zhao, X. et al. Engineering amphiphilic membrane surfaces based on PEO and PDMS segments for improved antifouling performances. J. Membr. Sci. 450, 111–123 (2014).
Shaffer, D. L., Werber, J. R., Jaramillo, H., Lin, S. & Elimelech, M. Forward osmosis: where are we now? Desalination 356, 271–284 (2015).
Tiraferri, A., Yip, N. Y., Phillip, W. A., Schiffman, J. D. & Elimelech, M. Relating performance of thin-film composite forward osmosis membranes to support layer formation and structure. J. Membr. Sci. 367, 340–352 (2011).
Lu, X., Arias Chavez, L. H., Romero-Vargas Castrillón, S., Ma, J. & Elimelech, M. Influence of active layer and support layer surface structures on organic fouling propensity of thin-film composite forward osmosis membranes. Environ. Sci. Technol. 49, 1436–1444 (2015).
Pohl, P., Saparov, S. M., Borgnia, M. J. & Agre, P. Highly selective water channel activity measured by voltage clamp: analysis of planar lipid bilayers reconstituted with purified AqpZ. Proc. Natl Acad. Sci. USA 98, 9624–9629 (2001).
Ruiz, L., Wu, Y. & Keten, S. Tailoring the water structure and transport in nanotubes with tunable interiors. Nanoscale 7, 121–132 (2015).
Acknowledgements
The authors acknowledge support received from the US National Science Foundation (NSF) under award numbers CBET-1437630 and CMMI-1246804 and through the NSF Nanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment (ERC-1449500). The authors also acknowledge the NSF Graduate Research Fellowship awarded to J.R.W.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Werber, J., Osuji, C. & Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat Rev Mater 1, 16018 (2016). https://doi.org/10.1038/natrevmats.2016.18
Published:
DOI: https://doi.org/10.1038/natrevmats.2016.18
This article is cited by
-
Interplay of the forces governing steroid hormone micropollutant adsorption in vertically-aligned carbon nanotube membrane nanopores
Nature Communications (2024)
-
Development of multifunctional membranes via plasma-assisted nonsolvent induced phase separation
Nature Communications (2024)
-
Pressure-driven membrane desalination
Nature Reviews Methods Primers (2024)
-
A Molecular-Sieving Interphase Towards Low-Concentrated Aqueous Sodium-Ion Batteries
Nano-Micro Letters (2024)
-
Research Progress in the Fabrication of Covalent Organic Framework Membranes for Chemical Separations
Chinese Journal of Polymer Science (2024)