Abstract
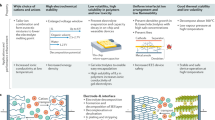
Salts that are liquid at room temperature, now commonly called ionic liquids, have been known for more than 100 years; however, their unique properties have only come to light in the past two decades. In this Review, we examine recent work in which the properties of ionic liquids have enabled important advances to be made in sustainable energy generation and storage. We discuss the use of ionic liquids as media for synthesis of electromaterials, for example, in the preparation of doped carbons, conducting polymers and intercalation electrode materials. Focusing on their intrinsic ionic conductivity, we examine recent reports of ionic liquids used as electrolytes in emerging high-energy-density and low-cost batteries, including Li-ion, Li–O2, Li–S, Na-ion and Al-ion batteries. Similar developments in electrolyte applications in dye-sensitized solar cells, thermo-electrochemical cells, double-layer capacitors and CO2 reduction are also discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Smiglak, M. et al. Ionic liquids for energy, materials, and medicine. Chem. Commun. 50, 9228–9250 (2014).
MacFarlane, D. R. et al. Energy applications of ionic liquids. Energy Environ. Sci. 7, 232–250 (2014).
Ohno, H. Electrochemical Aspects of Ionic Liquids 2nd edn (Wiley, 2011).
Pringle, J. M., Forsyth, M. & MacFarlane, D. R. in Electrodeposition from Ionic Liquids (eds Endres, F., Abbott, A & MacFarlane, D. R. ) 167–211 (Wiley-VCH, 2008).
Endres, F., MacFarlane, D. R. & Abbott, A. P. Electrodepositon from Ionic Liquids 1st edn (2008).
Sandoval, A. P., Feliu, J. M., Torresi, R. M. & Suarez-Herrera, M. F. Electrochemical properties of poly(3,4-ethylenedioxythiophene) grown on Pt(111) in imidazolium ionic liquids. RSC Adv. 4, 3383–3391 (2014).
Dobbelin, M., Marcilla, R., Pozo-Gonzalo, C. & Mecerreyes, D. Innovative materials and applications based on poly(3,4-ethylenedioxythiophene) and ionic liquids. J. Mater. Chem. 20, 7613–7622 (2010).
Abdelhamid, M. E., Snook, G. A. & O'Mullane, A. P. Electropolymerisation of catalytically active PEDOT from an ionic liquid on a flexible carbon cloth using a sandwich cell configuration. ChemPlusChem 80, 74–82 (2015).
Astratine, L., Magner, E., Cassidy, J. & Betts, A. Electrodeposition and characterisation of copolymers based on pyrrole and 3,4-ethylenedioxythiophene in BMIM BF4 using a microcell configuration. Electrochim. Acta 115, 440–448 (2014).
Wang, Z. P. et al. Poly(thieno[3,4-b]-1,4-oxathiane): medium effect on electropolymerization and electrochromic performance. Langmuir 30, 15581–15589 (2014).
Kannan, B., Williams, D. E., Laslau, C. & Travas-Sejdic, J. The electrochemical growth of highly conductive single PEDOT (conducting polymer):BMIPF6 (ionic liquid) nanowires. J. Mater. Chem. 22, 18132–18135 (2012).
Carstens, T., Prowald, A., El Abedin, S. Z. & Endres, F. Electrochemical synthesis of PEDOT and PPP macroporous films and nanowire architectures from ionic liquids. J. Solid State Electrochem. 16, 3479–3485 (2012).
Lagoutte, S. et al. Poly(3-methylthiophene)/vertically aligned multi-walled carbon nanotubes: electrochemical synthesis, characterizations and electrochemical storage properties in ionic liquids. Electrochim. Acta 130, 754–765 (2014).
Descroix, S., Hallais, G., Lagrost, C. & Pinson, J. Regular poly(para-phenylene) films bound to gold surfaces through the electrochemical reduction of diazonium salts followed by electropolymerization in an ionic liquid. Electrochim. Acta 106, 172–180 (2013).
Wallace, G. G., Moulton, S. E., Kapsa, R. M. I. & Higgins, M. J. Organic Bionics (Wiley-VCH, 2012).
Du, Z. J., Luo, X., Weaver, C. L. & Cui, X. T. Poly(3,4-ethylenedioxythiophene)-ionic liquid coating improves neural recording and stimulation functionality of MEAs. J. Mater. Chem. C 3, 6515–6524 (2015).
Fellinger, T. P., Thomas, A., Yuan, J. Y. & Antonietti, M. 25th anniversary article: ‘Cooking carbon with salt’: carbon materials and carbonaceous frameworks from ionic liquids and poly(ionic liquid)s. Adv. Mater. 25, 5838–5854 (2013).
Lee, J. S., Wang, X. Q., Luo, H. M., Baker, G. A. & Dai, S. Facile ionothermal synthesis of microporous and mesoporous carbons from task specific ionic liquids. J. Am. Chem. Soc. 131, 4596–4597 (2009).
Zhang, S. G., Dokko, K. & Watanabe, M. Carbon materialization of ionic liquids: from solvents to materials. Mater. Horiz. 2, 168–197 (2015).
Paraknowitsch, J. P., Zhang, J., Su, D., Thomas, A. & Antonietti, M. Ionic liquids as precursors for nitrogen-doped graphitic carbon. Adv. Mater. 22, 87–92 (2010).
Fulvio, P. F. et al. A new family of fluidic precursors for the self-templated synthesis of hierarchical nanoporous carbons. Chem. Commun. 49, 7289–7291 (2013).
Fellinger, T. P., Hasche, F., Strasser, P. & Antonietti, M. Mesoporous nitrogen-doped carbon for the electrocatalytic synthesis of hydrogen peroxide. J. Am. Chem. Soc. 134, 4072–4075 (2012).
Fechler, N., Fellinger, T. P. & Antonietti, M. ‘Salt templating’: a simple and sustainable pathway toward highly porous functional carbons from ionic liquids. Adv. Mater. 25, 75–79 (2013).
Zhang, S., Miran, M. S., Ikoma, A., Dokko, K. & Watanabe, M. Protic ionic liquids and salts as versatile carbon precursors. J. Am. Chem. Soc. 136, 1690–1693 (2014).
Zhang, S., Dokko, K. & Watanabe, M. Direct synthesis of nitrogen-doped carbon materials from protic ionic liquids and protic salts: structural and physicochemical correlations between precursor and carbon. Chem. Mater. 26, 2915–2926 (2014).
Zhang, S. G., Tsuzuki, S., Ueno, K., Dokko, K. & Watanabe, M. Upper limit of nitrogen content in carbon materials. Angew. Chem. Int. Ed. Engl. 54, 1302–1306 (2015).
Yang, W., Fellinger, T. P. & Antonietti, M. Efficient metal-free oxygen reduction in alkaline medium on high-surface-area mesoporous nitrogen-doped carbons made from ionic liquids and nucleobases. J. Am. Chem. Soc. 133, 206–209 (2011).
Elumeeva, K., Fechler, N., Fellinger, T. P. & Antonietti, M. Metal-free ionic liquid-derived electrocatalyst for high-performance oxygen reduction in acidic and alkaline electrolytes. Mater. Horiz. 1, 588–594 (2014).
Gong, K. P., Du, F., Xia, Z. H., Durstock, M. & Dai, L. M. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 323, 760–764 (2009).
Cooper, E. R. et al. Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature 430, 1012–1016 (2004).
Barpanda, P., Djellab, K., Recham, N., Armand, M. & Tarascon, J. M. Direct and modified ionothermal synthesis of LiMnPO4 with tunable morphology for rechargeable Li-ion batteries. J. Mater. Chem. 21, 10143–10152 (2011).
Duan, X., Ma, J., Lian, J. & Zheng, W. The art of using ionic liquids in the synthesis of inorganic nanomaterials. CrystEngComm 16, 2550–2559 (2014).
Eshetu, G. G., Armand, M., Scrosati, B. & Passerini, S. Energy storage materials synthesized from ionic liquids. Angew. Chem. Int. Ed. Engl. 53, 13342–13359 (2014).
Liu, P. I. et al. Microwave-assisted ionothermal synthesis of nanostructured anatase titanium dioxide/activated carbon composite as electrode material for capacitive deionization. Electrochim. Acta 96, 173–179 (2013).
Teng, F. et al. Synergism of ionic liquid and surfactant molecules in the growth of LiFePO4 nanorods and the electrochemical performances. J. Power Sources 202, 384–388 (2012).
Xiao, Z. H., Cui, Q. Q., Li, X. L., Wang, H. L. & Zhou, Q. Ionothermal synthesis for Mg-doped LiMn1.5Ni0.5O4 spinel with structural stability and high-rate performance. Ionics 21, 1261–1267 (2015).
Duan, X. C. et al. Ionic liquid-modulated preparation of hexagonal tungsten trioxide mesocrystals for lithium-ion batteries. Nanoscale 7, 2230–2234 (2015).
Li, C. L., Yin, C. L., Mu, X. K. & Maier, J. Top-down synthesis of open framework fluoride for lithium and sodium batteries. Chem. Mater. 25, 962–969 (2013).
Jana, M. K., Rajendra, H. B., Bhattacharyya, A. J. & Biswas, K. Green ionothermal synthesis of hierarchical nanostructures of SnS2 and their Li-ion storage properties. CrystEngComm 16, 3994–4000 (2014).
Mali, S. S., Betty, C. A., Bhosale, P. N., Patil, P. S. & Hong, C. K. From nanocorals to nanorods to nanoflowers nanoarchitecture for efficient dye-sensitized solar cells at relatively low film thickness: all hydrothermal process. Sci. Rep. 4, 5451 (2014).
Chatel, G. & MacFarlane, D. R. Ionic liquids and ultrasound in combination: synergies and challenges. Chem. Soc. Rev. 43, 8132–8149 (2014).
Choi, B. G. et al. Enhanced pseudocapacitance of ionic liquid/cobalt hydroxide nanohybrids. ACS Nano 7, 2453–2460 (2013).
Lahiri, A., Olschewski, M., Carstens, T., Abedin, S. Z. & Endres, F. Electrodeposition of crystalline gallium-doped germanium and Six Ge1−x from an ionic liquid at room temperature. ChemElectroChem 2, 571–577 (2015).
Izgorodin, A., Izgorodina, E. & MacFarlane, D. R. Low overpotential water oxidation to hydrogen peroxide on a MnOx catalyst. Energy Environ. Sci. 5, 9496–9501 (2012).
Zhou, F. et al. Enhanced photo-electrochemical water oxidation on MnOx in buffered organic/inorganic electrolytes. J. Mater. Chem. A 3, 16642–16652 (2015).
Asnavandi, M., Suryanto, B. H. R. & Zhao, C. Controlled electrodeposition of nanostructured Pd thin films from protic ionic liquids for electrocatalytic oxygen reduction reactions. RSC Adv. 5, 74017–74023 (2015).
Shrestha, S. & Biddinger, E. J. Palladium electrodeposition in 1-butyl-1-methylpyrrolidinium dicyanamide ionic liquid. Electrochim. Acta 174, 254–263 (2015).
Murugesan, S. et al. Room temperature electrodeposition of molybdenum sulfide for catalytic and photoluminescence applications. ACS Nano 7, 8199–8205 (2013).
Serrà, A., Gómez, E. & Vallés, E. Novel electrodeposition media to synthesize CoNi-Pt Core@Shell stable mesoporous nanorods with very high active surface for methanol electro-oxidation. Electrochim. Acta 174, 630–639 (2015).
Kalhoff, J., Eshetu, G. G., Bresser, D. & Passerini, S. Safer electrolytes for lithium-ion batteries: state of the art and perspectives. ChemSusChem 8, 2154–2175 (2015).
Ponrouch, A. et al. Towards high energy density sodium ion batteries through electrolyte optimization. Energy Environ. Sci. 6, 2361–2369 (2013).
Scheers, J., Fantini, S. & Johansson, P. A review of electrolytes for lithium–sulphur batteries. J. Power Sources 255, 204–218 (2014).
Rosenman, A. et al. Review on Li–sulfur battery systems: an integral perspective. Adv. Energy Mater. 5, 1500212 (2015).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J. M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 11, 19–29 (2012).
Shao, Y. Y. et al. Making Li–air batteries rechargeable: material challenges. Adv. Funct. Mater. 23, 987–1004 (2013).
Kar, M., Simons, T. J., Forsyth, M. & MacFarlane, D. R. Ionic liquid electrolytes as a platform for rechargeable metal–air batteries: a perspective. Phys. Chem. Chem. Phys. 16, 18658–18674 (2014).
Elia, G. A. et al. An advanced lithium–air battery exploiting an ionic liquid-based electrolyte. Nano Lett. 14, 6572–6577 (2014).
Wu, F. et al. Ionic liquid electrolytes with protective lithium difluoro(oxalate)borate for high voltage lithium-ion batteries. Nano Energy 13, 546–553 (2015).
Wongittharom, N. et al. Ionic liquid electrolytes for high-voltage rechargeable Li/LiNi0.5Mn1.5O4 cells. J. Mater. Chem. A 2, 3613–3620 (2014).
Di Lecce, D., Brutti, S., Panero, S. & Hassoun, J. A new Sn-C/LiFe0.1Co0.9PO4 full lithium-ion cell with ionic liquid-based electrolyte. Mater. Lett. 139, 329–332 (2015).
Girard, G. M. A. et al. Electrochemical and physicochemical properties of small phosphonium cation ionic liquid electrolytes with high lithium salt content. Phys. Chem. Chem. Phys. 17, 8706–8713 (2015).
Tsunashima, K., Sakai, Y. & Matsumiya, M. Physical and electrochemical properties of phosphonium ionic liquids derived from trimethylphosphine. Electrochem. Commun. 39, 30–33 (2014).
Scarbath-Evers, L. K., Hunt, P. A., Kirchner, B., MacFarlane, D. R. & Zahn, S. Molecular features contributing to the lower viscosity of phosphonium ionic liquids compared to their ammonium analogues. Phys. Chem. Chem. Phys. 17, 20205–20216 (2015).
Yoon, H. et al. Lithium electrochemistry and cycling behaviour of ionic liquids using cyano based anions. Energy Environ. Sci. 6, 979–986 (2013).
Singh, R. P., Martin, J. L. & Poshusta, J. C. Synthesis of bis(fluorosulfonyl)imide. US Patent 8377406 (2013).
Lahiri, A., Schubert, T. J. S., Iliev, B. & Endres, F. LiTFSI in 1-butyl-1-methylpyrrolidinium bis(fluorosulfonyl)amide: a possible electrolyte for ionic liquid based lithium ion batteries. Phys. Chem. Chem. Phys. 17, 11161–11164 (2015).
Matsui, Y. et al. Charge–discharge characteristics of a LiNi1/3Mn1/3Co1/3O2 cathode in FSI-based ionic liquids. Electrochemistry 80, 808–811 (2012).
Yoon, H., Howlett, P. C., Best, A. S., Forsyth, M. & MacFarlane, D. R. Fast charge/discharge of Li metal batteries using an ionic liquid electrolyte. J. Electrochem. Soc. 160, A1629–A1637 (2013).
Yoon, H., Best, A. S., Forsyth, M., MacFarlane, D. R. & Howlett, P. C. Physical properties of high Li-ion content N-propyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide based ionic liquid electrolytes. Phys. Chem. Chem. Phys. 17, 4656–4663 (2015).
Grande, L., Paillard, E., Kim, G. T., Monaco, S. & Passerini, S. Ionic liquid electrolytes for Li–air batteries: lithium metal cycling. Int. J. Mol. Sci. 15, 8122–8137 (2014).
Jang, I.-C., Ida, S. & Ishihara, T. Lithium depletion and the rechargeability of Li–O2 batteries in ether and carbonate electrolytes. ChemElectroChem 2, 1380–1384 (2015).
Piper, D. M. et al. Stable silicon–ionic liquid interface for next-generation lithium-ion batteries. Nat. Commun. 6, 6230 (2015).
Barghamadi, M. et al. Lithium–sulfur batteries — the solution is in the electrolyte, but is the electrolyte a solution? Energy Environ. Sci. 7, 3902–3920 (2014).
Zhang, S., Ueno, K., Dokko, K. & Watanabe, M. Recent advances in electrolytes for lithium–sulfur batteries. Adv. Energy Mater. 5, 1500117 (2015).
Park, J.-W., Ueno, K., Tachikawa, N., Dokko, K. & Watanabe, M. Ionic liquid electrolytes for lithium–sulfur batteries. J. Phys. Chem. C 117, 20531–20541 (2013).
Xiong, S. Z., Xie, K., Blomberg, E., Jacobsson, P. & Matic, A. Analysis of the solid electrolyte interphase formed with an ionic liquid electrolyte for lithium–sulfur batteries. J. Power Sources 252, 150–155 (2014).
Ueno, K. et al. Glyme–lithium salt equimolar molten mixtures: concentrated solutions or solvate ionic liquids? J. Phys. Chem. B 116, 11323–11331 (2012).
Li, F. J., Zhang, T., Yamada, Y., Yamada, A. & Zhou, H. S. Enhanced cycling performance of Li–O2 batteries by the optimized electrolyte concentration of LiTFSA in Glymes. Adv. Energy Mater. 3, 532–538 (2013).
Suo, L. M., Hu, Y. S., Li, H., Armand, M. & Chen, L. Q. A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 4, 1481 (2013).
Qian, J. F. et al. High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 6362 (2015).
Lu, Y. Y., Korf, K., Kambe, Y., Tu, Z. Y. & Archer, L. A. Ionic-liquid–nanoparticle hybrid electrolytes: applications in lithium metal batteries. Angew. Chem. Int. Ed. Engl. 53, 488–492 (2014).
Guyomard-Lack, A. et al. Hybrid silica–polymer ionogel solid electrolyte with tunable properties. Adv. Energy Mater. 4, 1301570 (2014).
Jin, L. et al. An organic ionic plastic crystal electrolyte for rate capability and stability of ambient temperature lithium batteries. Energy Environ. Sci. 7, 3352–3361 (2014).
Howlett, P. C. et al. Thin and flexible solid-state organic ionic plastic crystal-polymer nanofibre composite electrolytes for device applications. Phys. Chem. Chem. Phys. 15, 13784–13789 (2013).
Iranipour, N. et al. Ionic transport through a composite structure of N-ethyl-N-methylpyrrolidinium tetrafluoroborate organic ionic plastic crystals reinforced with polymer nanofibres. J. Mater. Chem. A 3, 6038–6052 (2015).
Goodenough, J. B. Evolution of strategies for modern rechargeable batteries. Acc. Chem. Res. 46, 1053–1061 (2013).
Slater, M. D., Kim, D., Lee, E. & Johnson, C. S. Sodium-ion batteries. Adv. Funct. Mater. 23, 947–958 (2013).
Fukunaga, A. et al. Intermediate-temperature ionic liquid NaFSA-KFSA and its application to sodium secondary batteries. J. Power Sources 209, 52–56 (2012).
Mohd, N. S. A., Gunzelmann, D., Sun, J., MacFarlane, D. R. & Forsyth, M. Ion conduction and phase morphology in sulfonate copolymer ionomers based on ionic liquid-sodium cation mixtures. J. Mater. Chem. A 2, 365–374 (2014).
Noor, S. A. M., Yoon, H., Forsyth, M. & MacFarlane, D. R. Gelled ionic liquid sodium ion conductors for sodium batteries. Electrochim. Acta 169, 376–381 (2015).
Yoon, H. et al. Physicochemical properties of N-propyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide for sodium metal battery applications. Phys. Chem. Chem. Phys. 16, 12350–12355 (2014).
Chagas, L. G., Buchholz, D., Wu, L. M., Vortmann, B. & Passerini, S. Unexpected performance of layered sodium-ion cathode material in ionic liquid-based electrolyte. J. Power Sources 247, 377–383 (2014).
Ding, C. et al. Na[FSA]-[C3C1pyrr][FSA] ionic liquids as electrolytes for sodium secondary batteries: Effects of Na ion concentration and operation temperature. J. Power Sources 269, 124–128 (2014).
Wang, C.-H. et al. Rechargeable Na/Na0.44MnO2 cells with ionic liquid electrolytes containing various sodium solutes. J. Power Sources 274, 1016–1023 (2015).
Wongittharom, N., Wang, C. H., Wang, Y. C., Yang, C. H. & Chang, J. K. Ionic liquid electrolytes with various sodium solutes for rechargeable Na/NaFePO4 batteries operated at elevated temperatures. ACS Appl. Mater. Interfaces 6, 17564–17570 (2014).
Monti, D., Jonsson, E., Palacin, M. R. & Johansson, P. Ionic liquid based electrolytes for sodium-ion batteries: Na+ solvation and ionic conductivity. J. Power Sources 245, 630–636 (2014).
Chen, F. F., Pringle, J. M. & Forsyth, M. Insights into the transport of alkali metal ions doped into a plastic crystal electrolyte. Chem. Mater. 27, 2666–2672 (2015).
Poetz, S. et al. Evaluation of decomposition products of EMImCl·1.5AlCl3 during aluminium electrodeposition with different analytical methods. RSC Adv. 4, 6685–6690 (2014).
Wilkes, J. S., Levisky, J. A., Wilson, R. A. & Hussey, C. L. Dialkylimidazolium chloroaluminate melts: a new class of room-temperature ionic liquids for electrochemistry, spectroscopy, and synthesis. Inorg. Chem. 21, 1263–1264 (1982).
Lin, M. C. et al. An ultrafast rechargeable aluminium-ion battery. Nature 520, 325–328 (2015).
Fang, Y. et al. An AlCl3 based ionic liquid with a neutral substituted pyridine ligand for electrochemical deposition of aluminum. Electrochim. Acta 160, 82–88 (2015).
Kakibe, T., Hishii, J. Y., Yoshimoto, N., Egashira, M. & Morita, M. Binary ionic liquid electrolytes containing organo-magnesium complex for rechargeable magnesium batteries. J. Power Sources 203, 195–200 (2012).
Khoo, T. et al. Discharge behaviour and interfacial properties of a magnesium battery incorporating trihexyl(tetradecyl)phosphonium based ionic liquid electrolytes. Electrochim. Acta 87, 701–708 (2013).
Mohtadi, R., Matsui, M., Arthur, T. S. & Hwang, S. J. Magnesium borohydride: from hydrogen storage to magnesium battery. Angew. Chem. Int. Ed. Engl. 51, 9780–9783 (2012).
Shao, Y. et al. Coordination chemistry in magnesium battery electrolytes: how ligands affect their performance. Sci. Rep. 3, 3130 (2013).
Kar, M. et al. Stable zinc cycling in novel alkoxy-ammonium based ionic liquid electrolytes. Electrochim. Acta 188, 461–471 (2016).
Forsyth, M., Howlett, P. C., Tan, S. K., MacFarlane, D. R. & Birbilis, N. An ionic liquid surface treatment for corrosion protection of magnesium alloy AZ31. Electrochem. Solid State Lett. 9, B52–B55 (2006).
Simons, T. J., Torriero, A. A. J., Howlett, P. C., MacFarlane, D. R. & Forsyth, M. High current density, efficient cycling of Zn2+ in 1-ethyl-3-methylimidazolium dicyanamide ionic liquid: the effect of Zn2+ salt and water concentration. Electrochem. Commun. 18, 119–122 (2012).
Simons, T. J., Pearson, A. K., Pas, S. J. & MacFarlane, D. R. The electrochemical cycling and electrodeposition of lead from 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ionic liquid. Electrochim. Acta 174, 712–720 (2015).
Simons, T. J., MacFarlane, D. R., Forsyth, M. & Howlett, P. C. Zn electrochemistry in 1-ethyl-3-methylimidazolium and N-butyl-N-methylpyrrolidinium dicyanamides: promising new rechargeable Zn battery electrolytes. ChemElectroChem 1, 1688–1697 (2014).
Kar, M., Winther-Jensen, B., Forsyth, M. & MacFarlane, D. R. Chelating ionic liquids for reversible zinc electrochemistry. Phys. Chem. Chem. Phys. 15, 7191–7197 (2013).
Kar, M., Winther-Jensen, B., Forsyth, M. & MacFarlane, D. R. Exploring zinc coordination in novel zinc battery electrolytes. Phys. Chem. Chem. Phys. 16, 10816–10822 (2014).
Friesen, C. A., Wolfe, D. & Johnson, P. B. Metal–air cell with ion exchange material. World Patent WO2012174558A1 (2012).
Friesen, C. A. & Hayes, J. Electrochemical cell, and particularly a cell with electrodeposited fuel. US Patent 8546028 (2013).
Mathew, S. et al. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 6, 242–247 (2014).
Bai, Y., Zhang, J., Wang, Y., Zhang, M. & Wang, P. Lithium-modulated conduction band edge shifts and charge-transfer dynamics in dye-sensitized solar cells based on a dicyanamide ionic liquid. Langmuir 27, 4749–4755 (2011).
Lau, G. P. S., Tsao, H. N., Zakeeruddin, S. M., Grätzel, M. & Dyson, P. J. Highly stable dye-sensitized solar cells based on novel 1,2,3-triazolium ionic liquids. ACS Appl. Mater. Interfaces 6, 13571–13577 (2014).
Armel, V. et al. Ionic liquid electrolyte porphyrin dye sensitised solar cells. Chem. Commun. 46, 3146–3148 (2010).
Yella, A. et al. Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science 334, 629–634 (2011).
Xu, D., Zhang, H., Chen, X. & Yan, F. Imidazolium functionalized cobalt tris(bipyridyl) complex redox shuttles for high efficiency ionic liquid electrolyte dye-sensitized solar cells. J. Mater. Chem. A 1, 11933–11941 (2013).
Chu, T.-C. et al. Ionic liquid with a dual-redox couple for efficient dye-sensitized solar cells. ChemSusChem 7, 146–153 (2014).
Zhao, J. et al. Solvent-free ionic liquid/poly(ionic liquid) electrolytes for quasi-solid-state dye-sensitized solar cells. J. Mater. Chem. 21, 7326–7330 (2011).
Zhang, Y. et al. Performance enhancement for quasi-solid-state dye-sensitized solar cells by using acid-oxidized carbon nanotube-based gel electrolytes. Electrochim. Acta 61, 185–190 (2012).
Lu, S. et al. Water-resistant, solid-state, dye-sensitized solar cells based on hydrophobic organic ionic plastic crystal electrolytes. Adv. Mater. 26, 1266–1271 (2014).
Armel, V., Forsyth, M., MacFarlane, D. R. & Pringle, J. M. Organic ionic plastic crystal electrolytes; a new class of electrolyte for high efficiency solid state dye-sensitized solar cells. Energy Environ. Sci. 4, 2234–2239 (2011).
Pringle, J. M. Recent progress in the development and use of organic ionic plastic crystal electrolytes. Phys. Chem. Chem. Phys. 15, 1339–1351 (2013).
Abraham, T. J., MacFarlane, D. R. & Pringle, J. M. High Seebeck coefficient redox ionic liquid electrolytes for thermal energy harvesting. Energy Environ. Sci. 6, 2639–2645 (2013).
Jiao, N., Abraham, T. J., MacFarlane, D. R. & Pringle, J. M. Ionic liquid electrolytes for thermal energy harvesting using a cobalt redox couple. J. Electrochem. Soc. 161, D3061–D3065 (2014).
Abraham, T. J., MacFarlane, D. R. & Pringle, J. M. Seebeck coefficients in ionic liquids–prospects for thermo-electrochemical cells. Chem. Commun. 47, 6260–6262 (2011).
Koerver, R., MacFarlane, D. R. & Pringle, J. M. Evaluation of electrochemical methods for determination of the Seebeck coefficient of redox electrolytes. Electrochim. Acta 184, 186–192 (2015).
Abraham, T. J., Tachikawa, N., MacFarlane, D. R. & Pringle, J. M. Investigation of the kinetic and mass transport limitations in thermoelectrochemical cells with different electrode materials. Phys. Chem. Chem. Phys. 16, 2527–2532 (2014).
Lazar, M. A., Al-Masri, D., MacFarlane, D. R. & Pringle, J. M. Enhanced thermal energy harvesting performance of a cobalt redox couple in ionic liquid–solvent mixtures. Phys. Chem. Chem. Phys. http://dx.doi.org/10.1039/C5CP04305K (2015).
Arbizzani, C. et al. Safe, high-energy supercapacitors based on solvent-free ionic liquid electrolytes. J. Power Sources 185, 1575–1579 (2008).
Bé guin, F., Presser, V., Balducci, A. & Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 26, 2219–2251 (2014).
Brandt, A. & Balducci, A. Theoretical and practical energy limitations of organic and ionic liquid-based electrolytes for high voltage electrochemical double layer capacitors. J. Power Sources 250, 343–351 (2014).
Ruch, P. W., Cericola, D., Foelske, A., Kötz, R. & Wokaun, A. A comparison of the aging of electrochemical double layer capacitors with acetonitrile and propylene carbonate-based electrolytes at elevated voltages. Electrochim. Acta 55, 2352–2357 (2010).
Uesugi, E., Goto, H., Eguchi, R., Fujiwara, A. & Kubozono, Y. Electric double-layer capacitance between an ionic liquid and few-layer graphene. Sci. Rep. 3, 1595 (2013).
Yang, X., Cheng, C., Wang, Y., Qiu, L. & Li, D. Liquid-mediated dense integration of graphene materials for compact capacitive energy storage. Science 341, 534–537 (2013).
Coadou, E. et al. Comparative study on performances of trimethyl-sulfonium and trimethyl-ammonium based ionic liquids in molecular solvents as electrolyte for electrochemical double layer capacitors. J. Phys. Chem. C 117, 10315–10325 (2013).
Pohlmann, S. et al. Azepanium-based ionic liquids as green electrolytes for high voltage supercapacitors. J. Power Sources 273, 931–936 (2015).
Pohlmann, S. & Balducci, A. A new conducting salt for high voltage propylene carbonate-based electrochemical double layer capacitors. Electrochim. Acta 110, 221–227 (2013).
Wolff, C., Jeong, S., Paillard, E., Balducci, A. & Passerini, S. High power, solvent-free electrochemical double layer capacitors based on pyrrolidinium dicyanamide ionic liquids. J. Power Sources 293, 65–70 (2015).
Faunce, T. et al. Artificial photosynthesis as a frontier technology for energy sustainability. Energy Environ. Sci. 6, 1074–1076 (2013).
Faunce, T. A. et al. Energy and environment policy case for a global project on artificial photosynthesis. Energy Environ. Sci. 6, 695–698 (2013).
Cokoja, M., Bruckmeier, C., Rieger, B., Herrmann, W. A. & Kuhn, F. E. Transformation of carbon dioxide with homogeneous transition-metal catalysts: a molecular solution to a global challenge? Angew. Chem. Int. Ed. Engl. 50, 8510–8537 (2011).
Ramdin, M., de Loos, T. W. & Vlugt, T. J. H. State-of-the-art of CO2 capture with ionic liquids. Ind. Eng. Chem. Res. 51, 8149–8177 (2012).
Sun, L., Ramesha, G. K., Kamat, P. V. & Brennecke, J. F. Switching the reaction course of electrochemical CO2 reduction with ionic liquids. Langmuir 30, 6302–6308 (2014).
Rosen, B. A. et al. Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science 334, 643–644 (2011).
Rosen, B. A. et al. In situ spectroscopic examination of a low overpotential pathway for carbon dioxide conversion to carbon monoxide. J. Phys. Chem. C 116, 15307–15312 (2012).
Watkins, J. D. & Bocarsly, A. B. Direct reduction of carbon dioxide to formate in high-gas-capacity ionic liquids at post-transition-metal electrodes. ChemSusChem 7, 284–290 (2014).
Cheek, G. T., Roeper, D. F., Pearson, W. & O’Grady, W. E. Electrochemical studies of imidazolium carboxylate adducts in a room-temperature ionic liquid. ECS Trans. 64, 161–169 (2014).
Bernardini, G., Wedd, A. G., Zhao, C. & Bond, A. M. Electrochemical probing of the photoreduction of molybdenum and tungsten Dawson-type polyoxometalates in molecular and ionic liquid media using water as an electron donor. Dalton Trans. 41, 9944–9954 (2012).
McDonnell-Worth, C. & MacFarlane, D. R. Ion effects in water oxidation to hydrogen peroxide. RSC Adv. 4, 30551–30557 (2014).
Zhao, S.-F., Horne, M., Bond, A. M. & Zhang, J. Electrocarboxylation of acetophenone in ionic liquids: the influence of proton availability on product distribution. Green Chem. 16, 2242–2251 (2014).
Zhang, S. et al. Ionic liquid-based green processes for energy production. Chem. Soc. Rev. 43, 7838–7869 (2014).
Yasuda, T. & Watanabe, M. Protic ionic liquids: fuel cell applications. MRS Bull. 38, 560–566 (2013).
Shironita, S., Karasuda, K., Sato, M. & Umeda, M. Feasibility investigation of methanol generation by CO2 reduction using Pt/C-based membrane electrode assembly for a reversible fuel cell. J. Power Sources 228, 68–74 (2013).
Miran, M. S., Yasuda, T., Susan, M. A. B. H., Dokko, K. & Watanabe, M. Binary protic ionic liquid mixtures as a proton conductor: high fuel cell reaction activity and facile proton transport. J. Phys. Chem. C 118, 27631–27639 (2014).
Díaz, M., Ortiz, A. & Ortiz, I. Progress in the use of ionic liquids as electrolyte membranes in fuel cells. J. Membrane Sci. 469, 379–396 (2014).
Ye, Y.-S., Rick, J. & Hwang, B.-J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 1, 2719–2743 (2013).
Luo, J. et al. 1,2,4-Triazolium perfluorobutanesulfonate as an archetypal pure protic organic ionic plastic crystal electrolyte for all-solid-state fuel cells. Energy Environ. Sci. 8, 1276–1291 (2015).
Hernández-Fernández, F. J. et al. Recent progress and perspectives in microbial fuel cells for bioenergy generation and wastewater treatment. Fuel Process. Technol. 138, 284–297 (2015).
Hernández-Fernández, F. J. et al. New application of supported ionic liquids membranes as proton exchange membranes in microbial fuel cell for waste water treatment. Chem. Eng. J. 279, 115–119 (2015).
Fujita, K. et al. Solubility and stability of cytochrome c in hydrated ionic liquids: effect of oxo acid residues and kosmotropicity. Biomacromolecules 8, 2080–2086 (2007).
Fujita, K., Nikawa, Y. & Ohno, H. Cold crystallisation behaviour of water molecules in ionic liquids as a screening method to evaluate biocompatibility of the hydrated ionic liquids. Chem. Commun. 49, 3257–3259 (2013).
Fujita, K., Murata, K., Masuda, M., Nakamura, N. & Ohno, H. Ionic liquids designed for advanced applications in bioelectrochemistry. RSC Adv. 2, 4018–4030 (2012).
Ohno, H., Fujita, K. & Kohno, Y. Is seven the minimum number of water molecules per ion pair for assured biological activity in ionic liquid–water mixtures? Phys. Chem. Chem. Phys. 17, 14454–14460 (2015).
Tamura, K., Nakamura, N. & Ohno, H. Cytochrome c dissolved in 1-allyl-3-methylimidazolium chloride type ionic liquid undergoes a quasi-reversible redox reaction up to 140 °C. Biotechnol. Bioeng. 109, 729–735 (2012).
Fujita, K., Nakamura, N., Igarashi, K., Samejima, M. & Ohno, H. Biocatalytic oxidation of cellobiose in an hydrated ionic liquid. Green Chem. 11, 351–354 (2009).
Acknowledgements
D.R.M. and M.F. thank the Australian Research Council for support from the Australian Laureate Fellowship programme and J.M.P. and P.C.H. for support from the Discovery Projects program and the Australian Centre for Electromaterials Science. This work was supported in part by the National Natural of Science Foundation of China (Grant Nos 21371101, 21421001), 111 Project (B12015) and MOE Innovation Team (IRT 13022) of China. H.O. acknowledges the financial support of the Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI, No. 26248049).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
MacFarlane, D., Forsyth, M., Howlett, P. et al. Ionic liquids and their solid-state analogues as materials for energy generation and storage. Nat Rev Mater 1, 15005 (2016). https://doi.org/10.1038/natrevmats.2015.5
Published:
DOI: https://doi.org/10.1038/natrevmats.2015.5
This article is cited by
-
High-performance lithium–sulfur batteries utilizing charged binder and solid-state ionogel electrolyte
Macromolecular Research (2024)
-
The origins of catalytic selectivity for the electrochemical conversion of carbon dioxide to methanol
Nano Research (2024)
-
The rational design of poly (inoic liquid) for 4.0 V graphene-based supercapacitors
Journal of Materials Science: Materials in Electronics (2024)
-
Theoretical Study on the Application of a Janus CoSTe Monolayer for Li-S Batteries
Journal of Electronic Materials (2023)
-
Ionic liquids meet lipid bilayers: a state-of-the-art review
Biophysical Reviews (2023)