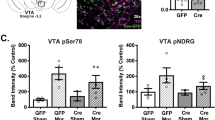
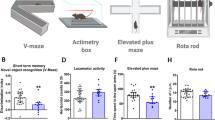
Abstract
Genetic factors significantly influence susceptibility for substance abuse and mood disorders. Rodent studies have begun to elucidate a role of Cav1.3 L-type Ca2+ channels in neuropsychiatric-related behaviors, such as addictive and depressive-like behaviors. Human studies have also linked the CACNA1D gene, which codes for the Cav1.3 protein, with bipolar disorder. However, the neurocircuitry and the molecular mechanisms underlying the role of Cav1.3 in neuropsychiatric phenotypes are not well established. In the present study, we directly manipulated Cav1.3 channels in Cav1.2 dihydropyridine insensitive mutant mice and found that ventral tegmental area (VTA) Cav1.3 channels mediate cocaine-related and depressive-like behavior through a common nucleus accumbens (NAc) shell calcium-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (CP-AMPAR) mechanism that requires GluA1 phosphorylation at S831. Selective activation of VTA Cav1.3 with (±)-BayK-8644 (BayK) enhanced cocaine conditioned place preference and cocaine psychomotor activity while inducing depressive-like behavior, an effect not observed in S831A phospho-mutant mice. Infusion of the CP-AMPAR-specific blocker Naspm into the NAc shell reversed the cocaine and depressive-like phenotypes. In addition, activation of VTA Cav1.3 channels resulted in social behavioral deficits. In contrast to the cocaine- and depression-related phenotypes, GluA1/A2 AMPARs in the NAc core mediated social deficits, independent of S831-GluA1 phosphorylation. Using a candidate gene analysis approach, we also identified single-nucleotide polymorphisms in the CACNA1D gene associated with cocaine dependence in human subjects. Together, our findings reveal novel, overlapping mechanisms through which VTA Cav1.3 mediates cocaine-related, depressive-like and social phenotypes, suggesting that Cav1.3 may serve as a target for the treatment of neuropsychiatric symptoms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Whitton AE, Treadway MT, Pizzagalli DA . Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry 2015; 28: 7–12.
Post RM, Kalivas P . Bipolar disorder and substance misuse: pathological and therapeutic implications of their comorbidity and cross-sensitisation. Br J Psychiatry 2013; 202: 172–176.
Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE . Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 617–627.
Kendler KS, Prescott CA, Myers J, Neale MC . The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry 2003; 60: 929–937.
Goldman D, Oroszi G, Ducci F . The genetics of addictions: uncovering the genes. Nat Rev Genet 2005; 6: 521–532.
Nestler EJ, Carlezon WA Jr. . The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 2006; 59: 1151–1159.
Luthi A, Luscher C . Pathological circuit function underlying addiction and anxiety disorders. Nat Neurosci 2014; 17: 1635–1643.
Russo SJ, Nestler EJ . The brain reward circuitry in mood disorders. Nat Rev Neurosci 2013; 14: 609–625.
Kabir ZD, Lee AS, Rajadhyaksha AM . L-type Ca2+ channels in mood, cognition and addiction: Integrating human and rodent studies with a focus on behavioural endophenotypes. J Physiol 2016; 594: 5823–5837.
Pinggera A, Striessnig J . Cav 1.3 (CACNA1D) L-type Ca2+ channel dysfunction in CNS disorders. J Physiol 2016; 594: 5839–5849.
Ament SA, Szelinger S, Glusman G, Ashworth J, Hou L, Akula N et al. Rare variants in neuronal excitability genes influence risk for bipolar disorder. Proc Natl Acad Sci USA 2015; 112: 3576–3581.
Ross J, Gedvilaite E, Badner JA, Erdman C, Baird L, Matsunami N et al. A rare variant in CACNA1D segregates with 7 bipolar i disorder cases in a large pedigree. Mol Neuropsychiatry 2016; 2: 145–150.
Pinggera A, Lieb A, Benedetti B, Lampert M, Monteleone S, Liedl KR et al. CACNA1D de novo mutations in autism spectrum disorders activate Cav1.3 L-type calcium channels. Biol Psychiatry 2015; 77: 816–822.
Licata SC, Freeman AY, Pierce-Bancroft AF, Pierce RC . Repeated stimulation of L-type calcium channels in the rat ventral tegmental area mimics the initiation of behavioral sensitization to cocaine. Psychopharmacology (Berl) 2000; 152: 110–118.
Schierberl K, Hao J, Tropea TF, Ra S, Giordano TP, Xu Q et al. Cav1.2 L-type Ca2+ channels mediate cocaine-induced GluA1 trafficking in the nucleus accumbens, a long-term adaptation dependent on ventral tegmental area Cav1.3 channels. J Neurosci 2011; 31: 13562–13575.
Degoulet M, Stelly CE, Ahn KC, Morikawa H . L-type Ca2+ channel blockade with antihypertensive medication disrupts VTA synaptic plasticity and drug-associated contextual memory. Mol Psychiatry 2016; 21: 394–402.
Stanika RI, Flucher BE, Obermair GJ . Regulation of postsynaptic stability by the L-type calcium channel Cav1.3 and its interaction with PDZ proteins. Curr Mol Pharmacol 2015; 8: 95–101.
Rajadhyaksha AM, Kosofsky BE . Psychostimulants, L-type calcium channels, kinases, and phosphatases. Neuroscientist 2005; 11: 494–502.
Pascoli V, Terrier J, Hiver A, Luscher C . Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron 2015; 88: 1054–1066.
Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2013; 493: 537–541.
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 2013; 493: 532–536.
Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A et al. Natural neural projection dynamics underlying social behavior. Cell 2014; 157: 1535–1551.
Liu Y, Dore J, Chen X . Calcium influx through L-type channels generates protein kinase M to induce burst firing of dopamine cells in the rat ventral tegmental area. J Biol Chem 2007; 282: 8594–8603.
Liu Y, Harding M, Pittman A, Dore J, Striessnig J, Rajadhyaksha A et al. Cav1.2 and Cav1.3 L-type calcium channels regulate dopaminergic firing activity in the mouse ventral tegmental area. J Neurophysiol 2014; 112: 1119–1130.
Covey DP, Roitman MF, Garris PA . Illicit dopamine transients: reconciling actions of abused drugs. Trends Neurosci 2014; 37: 200–210.
Walsh JJ, Han MH . The heterogeneity of ventral tegmental area neurons: projection functions in a mood-related context. Neuroscience 2014; 282: 101–108.
Rajadhyaksha A, Husson I, Satpute SS, Kuppenbender KD, Ren JQ, Guerriero RM et al. L-type Ca2+ channels mediate adaptation of extracellular signal-regulated kinase 1/2 phosphorylation in the ventral tegmental area after chronic amphetamine treatment. J Neurosci 2004; 24: 7464–7476.
Sinnegger-Brauns MJ, Hetzenauer A, Huber IG, Renström E, Wietzorrek G, Berjukov S et al. Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by L-type Ca2+ channels. J Clin Invest 2004; 113: 1430–1439.
Kourrich S, Rothwell PE, Klug JR, Thomas MJ . Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci 2007; 27: 7921–7928.
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 2008; 454: 118–121.
Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci 2009; 12: 1036–1041.
McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M . Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci 2011; 31: 5737–5743.
Pierce RC, Wolf ME . Psychostimulant-induced neuroadaptations in nucleus accumbens AMPA receptor transmission. Cold Spring Harb Perspect Med 2013; 3: a012021.
Pascoli V, Terrier J, Espallergues J, Valjent E, O'Connor EC, Luscher C . Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 2014; 509: 459–464.
Terrier J, Luscher C, Pascoli V . Cell-type specific insertion of GluA2-lacking AMPARs with cocaine exposure leading to sensitization, cue-induced seeking, and incubation of craving. Neuropsychopharmacology 2016; 41: 1779–1789.
Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC . Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 2012; 487: 183–189.
Hetzenauer A, Sinnegger-Brauns MJ, Striessnig J, Singewald N . Brain activation pattern induced by stimulation of L-type Ca2+-channels: contribution of Cav1.3 and Cav1.2 isoforms. Neuroscience 2006; 139: 1005–1015.
Giordano TP, Tropea TF, Satpute SS, Sinnegger-Brauns MJ, Striessnig J, Kosofsky BE et al. Molecular switch from L-type Cav1.3 to Cav1.2 Ca2+ channel signaling underlies long-term psychostimulant-induced behavioral and molecular plasticity. J Neurosci 2010; 30: 17051–17062.
Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H et al. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 2000; 102: 89–97.
Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 2003; 112: 631–643.
Tropea TF, Kabir ZD, Kaur G, Rajadhyaksha AM, Kosofsky BE . Enhanced dopamine D1 and BDNF signaling in the adult dorsal striatum but not nucleus accumbens of prenatal cocaine treated mice. Front Psychiatry 2011; 2: 67.
Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW . Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci 2010; 30: 7984–7992.
Calin-Jageman I, Yu K, Hall RA, Mei L, Lee A . Erbin enhances voltage-dependent facilitation of Cav1.3 Ca2+ channels through relief of an autoinhibitory domain in the Cav1.3 alpha1 subunit. J Neurosci 2007; 27: 1374–1385.
Tropea TF, Kosofsky BE, Rajadhyaksha AM . Enhanced CREB and DARPP-32 phosphorylation in the nucleus accumbens and CREB, ERK, and GluR1 phosphorylation in the dorsal hippocampus is associated with cocaine-conditioned place preference behavior. J Neurochem 2008; 106: 1780–1790.
Inan M, Zhao M, Manuszak M, Karakaya C, Rajadhyaksha AM, Pickel VM et al. Energy deficit in parvalbumin neurons leads to circuit dysfunction, impaired sensory gating and social disability. Neurobiol Dis 2016; 93: 35–46.
Lee AS, Ra S, Rajadhyaksha AM, Britt JK, De Jesus-Cortes H, Gonzales KL et al. Forebrain elimination of cacna1c mediates anxiety-like behavior in mice. Mol Psychiatry 2012; 17: 1054–1055.
Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, Pugh E et al. A genome-wide association study of alcohol dependence. Proc Natl Acad Sci USA 2010; 107: 5082–5087.
Huganir RL, Nicoll RA . AMPARs and synaptic plasticity: the last 25 years. Neuron 2013; 80: 704–717.
Goffer Y, Xu D, Eberle SE, D'Amour J, Lee M, Tukey D et al. Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J Neurosci 2013; 33: 19034–19044.
Li MX, Gui HS, Kwan JS, Sham PC . GATES: a rapid and powerful gene-based association test using extended Simes procedure. Am J Hum Genet 2011; 88: 283–293.
Polter AM, Kauer JA . Stress and VTA synapses: implications for addiction and depression. Eur J Neurosci 2014; 39: 1179–1188.
Busquet P, Nguyen NK, Schmid E, Tanimoto N, Seeliger MW, Ben-Yosef T et al. Cav1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int J Neuropsychopharmacol 2010; 13: 499–513.
Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci 2013; 16: 1644–1651.
Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 2014; 83: 1453–1467.
Wolf ME . Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci 2016; 17: 351–365.
Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT et al. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci 2014; 17: 73–80.
Vialou V, Robison AJ, Laplant QC, Covington HE 3rd, Dietz DM, Ohnishi YN et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci 2010; 13: 745–752.
Robison AJ, Vialou V, Mazei-Robison M, Feng J, Kourrich S, Collins M et al. Behavioral and structural responses to chronic cocaine require a feedforward loop involving DeltaFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J Neurosci 2013; 33: 4295–4307.
Robison AJ, Vialou V, Sun HS, Labonte B, Golden SA, Dias C et al. Fluoxetine epigenetically alters the CaMKIIalpha promoter in nucleus accumbens to regulate DeltaFosB binding and antidepressant effects. Neuropsychopharmacology 2014; 39: 1178–1186.
Easton AC, Lourdusamy A, Havranek M, Mizuno K, Solati J, Golub Y et al. alphaCaMKII controls the establishment of cocaine's reinforcing effects in mice and humans. Transl Psychiatry 2014; 4: e457.
Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ et al. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology 2011; 61: 1141–1151.
Araki R, Ago Y, Hasebe S, Nishiyama S, Tanaka T, Oka S et al. Involvement of prefrontal AMPA receptors in encounter stimulation-induced hyperactivity in isolation-reared mice. Int J Neuropsychopharmacol 2014; 17: 883–893.
Neuhofer D, Henstridge CM, Dudok B, Sepers M, Lassalle O, Katona I et al. Functional and structural deficits at accumbens synapses in a mouse model of Fragile X. Front Cell Neurosci 2015; 9: 100.
Taylor SR, Badurek S, Dileone RJ, Nashmi R, Minichiello L, Picciotto MR . GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J Comp Neurol 2014; 522: 3308–3334.
Kim CH, Chung HJ, Lee HK, Huganir RL . Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci USA 2001; 98: 11725–11730.
Seidenman KJ, Steinberg JP, Huganir R, Malinow R . Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci 2003; 23: 9220–9228.
States BA, Khatri L, Ziff EB . Stable synaptic retention of serine-880-phosphorylated GluR2 in hippocampal neurons. Mol Cell Neurosci 2008; 38: 189–202.
Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ et al. Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J Neurosci 2005; 25: 5553–5562.
Ghosh A, Carnahan J, Greenberg ME . Requirement for BDNF in activity-dependent survival of cortical neurons. Science 1994; 263: 1618–1623.
Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME . Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 1998; 20: 709–726.
Hong EJ, McCord AE, Greenberg ME . A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron 2008; 60: 610–624.
West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ et al. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA 2001; 98: 11024–11031.
Li X, Wolf ME . Multiple faces of BDNF in cocaine addiction. Behav Brain Res 2015; 279: 240–254.
Wook Koo J, Labonte B, Engmann O, Calipari ES, Juarez B, Lorsch Z et al. Essential role of mesolimbic brain-derived neurotrophic factor in chronic social stress-induced depressive behaviors. Biol Psychiatry 2015; 80: 469–478.
Caldeira MV, Melo CV, Pereira DB, Carvalho R, Correia SS, Backos DS et al. Brain-derived neurotrophic factor regulates the expression and synaptic delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J Biol Chem 2007; 282: 12619–12628.
Reimers JM, Loweth JA, Wolf ME . BDNF contributes to both rapid and homeostatic alterations in AMPA receptor surface expression in nucleus accumbens medium spiny neurons. Eur J Neurosci 2014; 39: 1159–1169.
Li X, Wolf ME . Brain-derived neurotrophic factor rapidly increases AMPA receptor surface expression in rat nucleus accumbens. Eur J Neurosci 2011; 34: 190–198.
McGinty JF, Whitfield TW Jr., Berglind WJ . Brain-derived neurotrophic factor and cocaine addiction. Brain Res 2010; 1314: 183–193.
Zamponi GW . Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat Rev Drug Discov 2016; 15: 19–34.
Acknowledgements
This work was supported by the National Institute of Health Grants R01DA029122 (to AMR), R01AA022994 (to SH), R21DA038048 (to NAA and AMR), NINDS R01NS036715 (to RLH), DC009433 and NS084190 (to AL), the NIDA Diversity Supplement 3R01DA029122-04S2 (to AM-R), The Weill Cornell Autism Research Program (to AMR), and the Austrian Science Fund FWF P-27809 (to JS). The Study of Addiction: Genetics and Environment (SAGE) GWAS data set used for the analyses described in this manuscript were obtained from http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gap through dbGaP accession number phs000092.v1.p. Funding support for the SAGE was provided through the NIH Genes, Environment and Health Initiative (GEI) (U01 HG004422). SAGE is one of the genome-wide association studies funded as part of the Gene Environment Association Studies (GENEVA) under GEI. Assistance with phenotype harmonization and genotype cleaning, as well as with general study coordination, was provided by the GENEVA Coordinating Center (U01 HG004446). Assistance with data cleaning was provided by the National Center for Biotechnology Information. Support for the collection of data sets and samples was provided by the Collaborative Study on the Genetics of Alcoholism (COGA; U10 AA008401), the Collaborative Genetic Study of Nicotine Dependence (COGEND; P01 CA089392) and the Family Study of Cocaine Dependence (FSCD; R01 DA013423). Funding support for genotyping, which was performed at the Johns Hopkins University Center for Inherited Disease Research, was provided by the NIH GEI (U01HG004438), the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse and the NIH contract ‘High throughput genotyping for studying the genetic contributions to human disease’ (HHSN268200782096C).
Author contributions
AM-R, JH, TFT, TPG, NAA and AMR contributed to the overall experimental design and data interpretation. AM-R, NAA and AMR wrote the manuscript. AL, RLH and JS provided reagents and edited the manuscript. AM-R, JH, TFT, TPG, MK and RCR performed research and analyzed data. MK and RCR edited the manuscript. SH contributed to the design and performed the GWAS and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Martínez-Rivera, A., Hao, J., Tropea, T. et al. Enhancing VTA Cav1.3 L-type Ca2+ channel activity promotes cocaine and mood-related behaviors via overlapping AMPA receptor mechanisms in the nucleus accumbens. Mol Psychiatry 22, 1735–1745 (2017). https://doi.org/10.1038/mp.2017.9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.9
This article is cited by
-
LPA1 receptors in the lateral habenula regulate negative affective states associated with alcohol withdrawal
Neuropsychopharmacology (2023)
-
Regulation of N-type calcium channels by nociceptin receptors and its possible role in neurological disorders
Molecular Brain (2022)
-
Reflex memory theory of acquired involuntary motor and sensory disorders
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2021)
-
De novo CACAN1D Ca2+ channelopathies: clinical phenotypes and molecular mechanism
Pflügers Archiv - European Journal of Physiology (2020)
-
The molecular and cellular mechanisms of depression: a focus on reward circuitry
Molecular Psychiatry (2019)