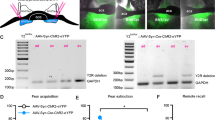
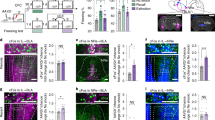
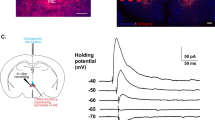
Abstract
The lateral central nucleus of the amygdala (CeAL) and the dorsolateral bed nucleus of the stria terminalis (BNSTDL) coordinate the expression of shorter- and longer-lasting fears, respectively. Less is known about how these structures communicate with each other during fear acquisition. One pathway, from the CeAL to the BNSTDL, is thought to communicate via corticotropin-releasing factor (CRF), but studies have yet to examine its function in fear learning and memory. Thus, we developed an adeno-associated viral-based strategy to selectively target CRF neurons with the optogenetic silencer archaerhodopsin tp009 (CRF-ArchT) to examine the role of CeAL CRF neurons and projections to the BNSTDL during the acquisition of contextual fear. Expression of our CRF-ArchT vector injected into the amygdala was restricted to CeAL CRF neurons. Furthermore, CRF axonal projections from the CeAL clustered around BNSTDL CRF cells. Optogenetic silencing of CeAL CRF neurons during contextual fear acquisition disrupted retention test freezing 24 h later, but only at later time points (>6 min) during testing. Silencing CeAL CRF projections in the BNSTDL during contextual fear acquisition produced a similar effect. Baseline contextual freezing, the rate of fear acquisition, freezing in an alternate context after conditioning and responsivity to foot shock were unaffected by optogenetic silencing. Our results highlight how CeAL CRF neurons and projections to the BNSTDL consolidate longer-lasting components of a fear memory. Our findings have implications for understanding how discrete amygdalar CRF pathways modulate longer-lasting fear in anxiety- and trauma-related disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Davis M, Walker DL, Miles L, Grillon C . Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 2010; 35: 105–135.
Herrmann MJ, Boehme S, Becker MP, Tupak SV, Guhn A, Schmidt B et al. Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Hum Brain Mapp 2015; 37: 1091–1102.
Münsterkötter AL, Notzon S, Redlich R, Grotegerd D, Dohm K, Arolt V et al. Spider or no spider? Neural correlates of sustained and phasic fear in spider phobia. Depress Anxiety 2015; 32: 656–663.
Yassa MA, Hazlett RL, Stark CE, Hoehn-Saric R . Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. J Psychiatr Res 2012; 46: 1045–1052.
Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 2012; 13: 769–787.
Lebow M, Chen A . Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry 2016; 21: 450–463.
Keck ME, Holsboer F . Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides 2001; 22: 835–844.
Vale W, Spiess J, Rivier C, Rivier J . Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981; 213: 1394–1397.
Bale TL, Vale WW . CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 2004; 44: 525–557.
Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K et al. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci USA 1994; 91: 8777–8781.
Swerdlow NR, Britton KT, Koob GF . Potentiation of acoustic startle by corticotropin-releasing factor (CRF) and by fear are both reversed by a-helical CRF (9-41). Neuropsychopharmacology 1989; 2: 285–292.
Liang K, Melia K, Campeau S, Falls W, Miserendino M, Davis M . Lesions of the central nucleus of the amygdala, but not the paraventricular nucleus of the hypothalamus, block the excitatory effects of corticotropin-releasing factor on the acoustic startle reflex. J Neurosci 1992; 12: 2313–2320.
Swerdlow N, Geyer MA, Vale W, Koob G . Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by chlordiazepoxide. Psychopharmacology 1986; 88: 147–152.
Gafford G, Ressler K . Mouse models of fear-related disorders: cell-type-specific manipulations in amygdala. Neuroscience 2016; 321: 108–120.
McCullough K, Morrison F, Ressler K . Bridging the gap: towards a cell-type specific understanding of neural circuits underlying fear behaviors. Neurobiol Learn Memory 2016; 135: 27–39.
Pitts MW, Takahashi LK . The central amygdala nucleus via corticotropin-releasing factor is necessary for time-limited consolidation processing but not storage of contextual fear memory. Neurobiol Learn Memory 2011; 95: 86–91.
Regev L, Tsoory M, Gil S, Chen A . Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biol Psychiatry 2012; 71: 317–326.
Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD et al. A central amygdala CRF circuit facilitates learning about weak threats. Neuron 2017; 93: 164–178.
Sakanaka M, Shibasaki T, Lederis K . Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res 1986; 382: 213–238.
Swanson LW, Sawchenko PE, Rivier J, Vale WW . Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 1983; 36: 165–186.
Walker DL, Davis M . Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct 2008; 213: 29–42.
Walker DL, Toufexis DJ, Davis M . Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 2003; 463: 199–216.
Morin S, Ling N, Liu X-J, Kahl S, Gehlert D . Differential distribution of urocortin-and corticotropin-releasing factor-like immunoreactivities in the rat brain. Neuroscience 1999; 92: 281–291.
Dabrowska J, Hazra R, Guo J-D, DeWitt S, Rainnie DG . Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front Neurosci 2013; 7: 156.
Sullivan G, Apergis J, Bush D, Johnson LR, Hou M, Ledoux J . Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience 2004; 128: 7–14.
Gafford GM, Ressler KJ . GABA and NMDA receptors in CRF neurons have opposing effects in fear acquisition and anxiety in central amygdala vs. bed nucleus of the stria terminalis. Horm Behav 2015; 76: 136–142.
Gungor NZ, Yamamoto R, Pare D . Optogenetic study of the projections from the bed nucleus of the stria terminalis to the central amygdala. J Neurophysiol 2015; 114: 2903–2911.
Martin EI, Ressler KJ, Binder E, Nemeroff CB . The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr Clin North Am 2009; 32: 549–575.
Duvarci S, Bauer EP, Paré D . The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J Neurosci 2009; 29: 10357–10361.
Hammack SE, Todd TP, Kocho-Schellenberg M, Bouton ME . Role of the bed nucleus of the stria terminalis in the acquisition of contextual fear at long or short context-shock intervals. Behav Neurosci 2015; 129: 673–678.
Walker DL, Davis M . Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci 1997; 17: 9375–9383.
Lee Y, Davis M . Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci 1997; 17: 6434–6446.
Asok A, Ayers LW, Awoyemi B, Schulkin J, Rosen JB . Immediate early gene and neuropeptide expression following exposure to the predator odor 2, 5-dihydro-2, 4, 5-trimethylthiazoline (TMT). Behav Brain Res 2013; 248: 85–93.
Root DH, Mejias-Aponte CA, Zhang S, Wang H-L, Hoffman AF, Lupica CR et al. Single rodent mesohabenular axons release glutamate and GABA. Nat Neurosci 2014; 17: 1543–1551.
Abdi H . Holm’s sequential Bonferroni procedure. In: Neil S (ed). Encyclopedia of Research Design. Thousand Oaks: CA, Sage, 2010.
Gaetano J . Holm-Bonferroni sequential correction: An EXCEL calculator (1.1) [Microsoft Excel workbook], 2013. Retrieved from https://www.researchgate.net/publication/236969037_Holm-Bonferroni_Sequential_Correction_An_EXCEL_Calculator.
Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci 2011; 5: 18.
Palkovits M, Brownstein MJ, Vale W . Distribution of corticotropin-releasing factor in rat brain. Fed Proc 1985; 44: 215–219.
Cusulin JIW, Füzesi T, Watts AG, Bains JS . Characterization of corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus of Crh-IRES-Cre mutant mice. PLoS ONE 2013; 8: e64943.
Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A et al. A transgenic rat for investigating the anatomy and function of corticotrophin releasing factor circuits. Front Neurosci 2015; 9: 487.
Chalmers DT, Lovenberg TW, De Souza EB . Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci 1995; 15: 6340–6350.
Asok A, Schulkin J, Rosen JB . Corticotropin releasing factor type-1 receptor antagonism in the dorsolateral bed nucleus of the stria terminalis disrupts contextually conditioned fear, but not unconditioned fear to a predator odor. Psychoneuroendocrinology 2016; 70: 17–24.
Holehonnur R, Luong JA, Chaturvedi D, Ho A, Lella SK, Hosek MP et al. Adeno-associated viral serotypes produce differing titers and differentially transduce neurons within the rat basal and lateral amygdala. BMC Neurosci 2014; 15: 1.
Nathanson JL, Yanagawa Y, Obata K, Callaway EM . Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience 2009; 161: 441–450.
Li D-P . Identification of corticotropin-releasing hormone neurons in paraventricular nucleus in rats (876.6). FASEB J 2014; 28 (1 Supplement): 876.
Przybycien-Szymanska MM, Mott NN, Pak TR . Alcohol dysregulates corticotropin-releasing-hormone (CRH) promoter activity by interfering with the negative glucocorticoid response element (nGRE). PLoS ONE 2011; 6: e26647.
Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 2012; 73: 553–566.
Jasnow AM, Rainnie DG, Maguschak KA, Chhatwal JP, Ressler KJ . Construction of cell-type specific promoter lentiviruses for optically guiding electrophysiological recordings and for targeted gene delivery. Methods Mol Biol 2009; 515: 199–213.
Chen Y, Molet J, Gunn BG, Ressler K, Baram TZ . Diversity of reporter expression patterns in transgenic mouse lines targeting corticotropin-releasing hormone-expressing neurons. Endocrinology 2015; 156: 4769–4780.
Andero R, Daniel S, Guo J-D, Bruner RC, Seth S, Marvar PJ et al. Amygdala-dependent molecular mechanisms of the Tac2 pathway in fear learning. Neuropsychopharmacology 2016; 41: 2714–2722.
Tovote P, Fadok JP, Lüthi A . Neuronal circuits for fear and anxiety. Nat Rev Neurosci 2015; 16: 317–331.
Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 2010; 468: 277–282.
Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 2010; 468: 270–276.
Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B . Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci 2013; 16: 332–339.
Yu K, da Silva PG, Albeanu DF, Li B . Central amygdala somatostatin neurons gate passive and active defensive behaviors. J Neurosci 2016; 36: 6488–6496.
Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE . Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of pavlovian fear conditioning. J Neurosci 2006; 26: 12387–12396.
Pitts MW, Todorovic C, Blank T, Takahashi LK . The central nucleus of the amygdala and corticotropin-releasing factor: insights into contextual fear memory. J Neurosci 2009; 29: 7379–7388.
Goosens KA, Maren S . Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem 2001; 8: 148–155.
El-Gaby M, Zhang Y, Wolf K, Schwiening CJ, Paulsen O, Shipton OA . Archaerhodopsin selectively and reversibly silences synaptic transmission through altered pH. Cell Rep 2016; 16: 2259–2268.
Davis M, Walker DL . Role of bed nucleus of the stria terminalis and amygdala AMPA receptors in the development and expression of context conditioning and sensitization of startle by prior shock. Brain Struct Funct 2014; 219: 1969–1982.
Gafford GM, Guo J-D, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ . Cell-type specific deletion of GABA (A) α1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc Natl Acad Sci USA 2012; 109: 16330–16335.
Mahn M, Prigge M, Ron S, Levy R, Yizhar O . Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat Neurosci 2016; 19: 554–556.
Day HE, Curran EJ, Watson SJ, Akil H . Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin‐1 β. J Comp Neurol 1999; 413: 113–128.
Petrovich G, Swanson L . Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res 1997; 763: 247–254.
Davis M . The role of the amygdala in fear and anxiety. Annu Rev Neurosci 1992; 15: 353–375.
Rosen JB, Schulkin J . From normal fear to pathological anxiety. Psychol Rev 1998; 105: 325–350.
Gungor NZ, Paré D . Functional heterogeneity in the bed nucleus of the stria terminalis. J Neurosci 2016; 36: 8038–8049.
Shackman AJ, Fox AS . Contributions of the central extended amygdala to fear and anxiety. J Neurosci 2016; 36: 8050–8063.
Avery S, Clauss J, Blackford J . The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology 2016; 41: 126–141.
Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ . Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci USA 2002; 99: 13908–13913.
Acknowledgements
This work was supported by NIH grant R01HD07506603 to JBR, an APA grant to AA, and by the National Institute on Drug Abuse Intramural Research Program (AFH and CRL). Microscopy access was supported by grants from the NIH-NIGMS (P20 GM103446), the NSF (IIA-1301765) and the State of Delaware.
Author contributions
AA, JBR and JS designed the experiments; AA and AD performed the behavioral experiments. AA performed the molecular work. CRL and AFH performed the electrophysiology experiments. AA, JBR, JS, CRL and AFH analyzed the data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Asok, A., Draper, A., Hoffman, A. et al. Optogenetic silencing of a corticotropin-releasing factor pathway from the central amygdala to the bed nucleus of the stria terminalis disrupts sustained fear. Mol Psychiatry 23, 914–922 (2018). https://doi.org/10.1038/mp.2017.79
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.79
This article is cited by
-
A sex-specific role for the bed nucleus of the stria terminalis in proactive defensive behavior
Neuropsychopharmacology (2023)
-
Chronic oxytocin-driven alternative splicing of Crfr2α induces anxiety
Molecular Psychiatry (2023)
-
Brain-wide perception of the emotional valence of light is regulated by distinct hypothalamic neurons
Molecular Psychiatry (2022)
-
Genome-wide translational profiling of amygdala Crh-expressing neurons reveals role for CREB in fear extinction learning
Nature Communications (2020)
-
Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats
Nature Communications (2019)