Abstract
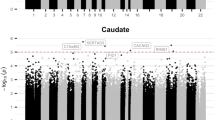
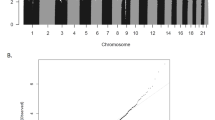
The study of monozygotic twins discordant for attention deficit hyperactivity disorder can elucidate mechanisms that contribute to the disorder, which affects ~7% of children. First, using in vivo neuroanatomic imaging on 14 pairs of monozygotic twins (mean age 9.7, s.d. 1.9 years), we found that discordance for the disorder is mirrored by differing dimensions of deep brain structures (the striatum and cerebellum), but not the cerebral cortex. Next, using whole-blood DNA from the same twins, we found a significant enrichment of epigenetic differences in genes expressed in these ‘discordant’ brain structures. Specifically, there is differential methylation of probes lying in the shore and shelf and enhancer regions of striatal and cerebellar genes. Notably, gene sets pertaining to the cerebral cortex (which did not differ in volume between affected and unaffected twins) were not enriched by differentially methylated probes. Genotypic differences between the twin pairs—such as copy number and rare, single-nucleotide variants—did not contribute to phenotypic discordance. Pathway analyses of the genes implicated by the most differentially methylated probes implicated γ-aminobutyric acid (GABA), dopamine and serotonin neurotransmitter systems. The study illustrates how neuroimaging can help guide the search for epigenomic mechanisms in neurodevelopmental disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
ADHD. Data and Statistics, 2011. Available at: http://www.cdc.gov/ncbddd/adhd/data.html (last accessed on 1 September 2016).
Hawi Z, Cummins T, Tong J, Johnson B, Lau R, Samarrai W et al. The molecular genetic architecture of attention deficit hyperactivity disorder. Mol Psychiatry 2015; 20: 289–297.
Franke B, Faraone SV, Asherson P, Buitelaar J, Bau CHD, Ramos-Quiroga JA et al. The genetics of attention deficit/hyperactivity disorder in adults, a review. Mol Psychiatry 2012; 17: 960–987.
Wong C, Meaburn EL, Ronald A, Price T, Jeffries A, Schalkwyk L et al. Methylomic analysis of monozygotic twins discordant for autism spectrum disorder and related behavioural traits. Mol Psychiatry 2014; 19: 495–503.
Cordova-Palomera A, Fatjo-Vilas M, Gasto C, Navarro V, Krebs M, Fananas L . Genome-wide methylation study on depression: differential methylation and variable methylation in monozygotic twins. Transl Psychiatry 2015; 5: e557.
Fisher HL, Murphy TM, Arseneault L, Caspi A, Moffitt TE, Viana J et al. Methylomic analysis of monozygotic twins discordant for childhood psychotic symptoms. Epigenetics 2015; 10: 1014–1023.
Dempster EL, Pidsley R, Schalkwyk LC, Owens S, Georgiades A, Kane F et al. Disease-associated epigenetic changes in monozygotic twins discordant for schizophrenia and bipolar disorder. Hum Mol Genet 2011; 20: 4786–4796.
Oh G, Wang S-C, Pal M, Chen ZF, Khare T, Tochigi M et al. DNA modification study of major depressive disorder: beyond locus-by-locus comparisons. Biol psychiatry 2015; 77: 246–255.
Wilmot B, Fry R, Smeester L, Musser ED, Mill J, Nigg JT . Methylomic analysis of salivary DNA in childhood ADHD identifies altered DNA methylation in VIPR2. J Child Psychol Psychiatry 2015; 57: 152–160.
Castellani CA, Awamleh Z, Melka MG, O'Reilly RL, Singh SM . Copy number variation distribution in six monozygotic twin pairs discordant for schizophrenia. Twin Res Hum Genet 2014; 17: 108–120.
Ono S, Imamura A, Tasaki S, Kurotaki N, Ozawa H, Yoshiura K-I et al. Failure to confirm CNVs as of aetiological significance in twin pairs discordant for schizophrenia. Twin Res Hum Genet 2010; 13: 455–460.
Bloom RJ, Kahler AK, Collins AL, Chen G, Cannon TD, Hultman C et al. Comprehensive analysis of copy number variation in monozygotic twins discordant for bipolar disorder or schizophrenia. Schizophr Res 2013; 146: 289–290.
Bruder CE, Piotrowski A, Gijsbers AA, Andersson R, Erickson S, de Ståhl TD et al. Phenotypically concordant and discordant monozygotic twins display different DNA copy-number-variation profiles. Am J Hum Genet 2008; 82: 763–771.
Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P et al. De novo mutations in schizophrenia implicate synaptic networks. Nature 2014; 506: 179–184.
Ronemus M, Iossifov I, Levy D, Wigler M . The role of de novo mutations in the genetics of autism spectrum disorders. Nat Rev Genet 2014; 15: 133–141.
Castellanos FX, Sharp WS, Gottesman RF, Greenstein DK, Giedd JN, Rapoport JL . Anatomic brain abnormalities in monozygotic twins discordant for attention deficit hyperactivity disorder. Am J Psychiatry 2003; 160: 1693–1696.
Nakao T, Radua J, Rubia K, Mataix-Cols D . Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry 2011; 2011: 24.
Valera EM, Faraone SV, Murray KE, Seidman LJ . Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry 2007; 61: 1361–1369.
Frodl T, Skokauskas N . Meta‐analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand 2012; 125: 114–126.
Xia S, Li X, Kimball AE, Kelly MS, Lesser I, Branch C . Thalamic shape and connectivity abnormalities in children with attention-deficit/hyperactivity disorder. Psychiatry Res 2012; 204: 161–167.
Castellanos FX, Proal E . Large-scale brain systems in ADHD: beyond the prefrontal–striatal model. Trends Cogn Sci 2012; 16: 17–26.
Reich W . Diagnostic interview for children and adolescents (DICA). J Am Acad Child Adolesc Psychiatry 2000; 39: 59–66.
Galwey NW . A new measure of the effective number of tests, a practical tool for comparing families of non‐independent significance tests. Genet Epidemiol 2009; 33: 559–568.
Morris TJ, Butcher LM, Feber A, Teschendorff AE, Chakravarthy AR, Wojdacz TK et al. ChAMP: 450k Chip Analysis Methylation Pipeline. Bioinformatics 2014; 30: 428–430.
Dempster EL, Pidsley R, Schalkwyk LC, Owens S, Georgiades A, Kane F et al. Disease-associated epigenetic changes in monozygotic twins discordant for schizophrenia and bipolar disorder. Hum Mol Genet 2011; 20: 4786–4796.
Jones PA . Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 2012; 13: 484–492.
Dempster EL, Wong CC, Lester KJ, Burrage J, Gregory AM, Mill J et al. Genome-wide methylomic analysis of monozygotic twins discordant for adolescent depression. Biol Psychiatry 2014; 76: 977–983.
Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M et al. Spatio-temporal transcriptome of the human brain. Nature 2011; 478: 483–489.
Elia J, Glessner JT, Wang K, Takahashi N, Shtir CJ, Hadley D et al. Genome-wide copy number variation study associates metabotropic glutamate receptor gene networks with attention deficit hyperactivity disorder. Nat Genet 2012; 44: 78–84.
Williams NM, Zaharieva I, Martin A, Langley K, Mantripragada K, Fossdal R et al. Rare chromosomal deletions and duplications in attention-deficit hyperactivity disorder: a genome-wide analysis. Lancet 2010; 376: 1401–1408.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009; 25: 2078–2079.
Hansen NF, Gartner JJ, Mei L, Samuels Y, Mullikin JC . Shimmer: detection of genetic alterations in tumors using next-generation sequence data. Bioinformatics 2013; 29: 1498–1503.
Larson DE, Harris CC, Chen K, Koboldt DC, Abbott TE, Dooling DJ et al. SomaticSniper: identification of somatic point mutations in whole genome sequencing data. Bioinformatics 2012; 28: 311–317.
Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 2013; 31: 213–219.
Wang K, Li M, Hakonarson H . ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010; 38: e164.
Lasky-Su J, Neale BM, Franke B, Anney RJL, Zhou K, Maller JB et al. Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am J Med Genet B 2008; 147B: 1345–1354.
Friedman LA, Rapoport JL . Brain development in ADHD. Curr Opin Neurobiol 2015; 30: 106–111.
Shaw P, De Rossi P, Watson B, Wharton A, Greenstein D, Raznahan A et al. Mapping the development of the basal ganglia in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2014; 53: 780–789.
Stoodley CJ . Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Front Systems Neurosci 2014; 8: 92.
Ivanov I . Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am J Psychiatry 2010; 167: 397.
Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF 3rd et al. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder [see comment]. Am J Psychiatry 2007; 164: 647–655.
Rossi R, Pievani M, Järvenpää T, Testa C, Koskenvuo M, Räihä I et al. Voxel‐based morphometry study on monozygotic twins discordant for Alzheimer's disease. Acta Neurol Scand 2015; 133: 427–433.
Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR . Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med 1990; 322: 789–794.
Pol HEH, Schnack HG, Mandl RC, Brans RG, van Haren NE, Baaré WF et al. Gray and white matter density changes in monozygotic and same-sex dizygotic twins discordant for schizophrenia using voxel-based morphometry. NeuroImage 2006; 31: 482–488.
Kates WR, Burnette CP, Eliez S, Strunge LA, Kaplan D, Landa R et al. Neuroanatomic variation in monozygotic twin pairs discordant for the narrow phenotype for autism. Am J Psychiatry 2004; 161: 539–546.
Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R . Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. J Child Psychol Psychiatry 2008; 49: 535–542.
Taurines R, Schwenck C, Westerwald E, Sachse M, Siniatchkin M, Freitag C . ADHD and autism: differential diagnosis or overlapping traits? A selective review. Atten Defic Hyperact Disord 2012; 4: 115–139.
Davies MN, Volta M, Pidsley R, Lunnon K, Dixit A, Lovestone S et al. Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome Biol 2012; 13: R43.
Kaminsky Z, Tochigi M, Jia P, Pal M, Mill J, Kwan A et al. A multi-tissue analysis identifies HLA complex group 9 gene methylation differences in bipolar disorder. Mol psychiatry 2012; 17: 728–740.
Lv J, Xin Y, Zhou W, Qiu Z . The epigenetic switches for neural development and psychiatric disorders. J Genet Genomics 2013; 40: 339–346.
Ma DK, Marchetto MC, Guo JU, Ming G-l, Gage FH, Song H . Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat Neurosci 2010; 13: 1338–1344.
Pidsley R, Dempster E, Mill J . Brain weight in males is correlated with DNA methylation at IGF2. Mol Psychiatry 2010; 15: 880–881.
Peña CJ, Bagot RC, Labonté B, Nestler EJ . Epigenetic signaling in psychiatric disorders. J Mol Biol 2014; 426: 3389–3412.
Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci 2002; 5: 308–315.
Graziano C, D'Elia AV, Mazzanti L, Moscano F, Guidelli Guidi S, Scarano E et al. A de novo nonsense mutation of PAX6 gene in a patient with aniridia, ataxia, and mental retardation. Am J Med Genet A 2007; 143A: 1802–1805.
Nakamura T, Jenkins NA, Copeland NG . Identification of a new family of Pbx-related homeobox genes. Oncogene 1996; 13: 2235–2242.
Matsui A, Tran M, Yoshida AC, Kikuchi SS, Mami U, Ogawa M et al. BTBD3 controls dendrite orientation toward active axons in mammalian neocortex. Science 2013; 342: 1114–1118.
Sofroniew MV, Howe CL, Mobley WC . Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 2001; 24: 1217–1281.
Ribases M, Hervas A, Ramos-Quiroga JA, Bosch R, Bielsa A, Gastaminza X et al. Association study of 10 genes encoding neurotrophic factors and their receptors in adult and child attention-deficit/hyperactivity disorder. Biol Psychiatry 2008; 63: 935–945.
Gassó P, Ortiz AE, Mas S, Morer A, Calvo A, Bargalló N et al. Association between genetic variants related to glutamatergic, dopaminergic and neurodevelopment pathways and white matter microstructure in child and adolescent patients with obsessive–compulsive disorder. J Affect Disord 2015; 186: 284–292.
Scott LJ, Muglia P, Kong XQ, Guan W, Flickinger M, Upmanyu R et al. Genome-wide association and meta-analysis of bipolar disorder in individuals of European ancestry. Proc Natl Acad Sci USA 2009; 106: 7501–7506.
Kunugi H, Hashimoto R, Yoshida M, Tatsumi M, Kamijima K . A missense polymorphism (S205L) of the low‐affinity neurotrophin receptor p75NTR gene is associated with depressive disorder and attempted suicide. Am J Med Genet B 2004; 129: 44–46.
Levy F, de Leon J . Dopamine ADHD/OCD theories: is glutamine part of the story? Neurotransmitter 2015; 2: e891; doi:10.14800/nt.891.
Nishi A, Bibb JA, Snyder GL, Higashi H, Nairn AC, Greengard P . Amplification of dopaminergic signaling by a positive feedback loop. Proc Natl Acad Sci USA 2000; 97: 12840–12845.
Krab LC, Goorden SM, Elgersma Y . Oncogenes on my mind: ERK and MTOR signaling in cognitive diseases. Trends Genet 2008; 24: 498–510.
Acknowledgements
The study was funded by the Intramural Research Programs of the National Human Genome Research Institute and the National Institute of Mental Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, YC., Sudre, G., Sharp, W. et al. Neuroanatomic, epigenetic and genetic differences in monozygotic twins discordant for attention deficit hyperactivity disorder. Mol Psychiatry 23, 683–690 (2018). https://doi.org/10.1038/mp.2017.45
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.45
This article is cited by
-
Cortico-striatal differences in the epigenome in attention-deficit/ hyperactivity disorder
Translational Psychiatry (2024)
-
Epigenome-wide association study identifies neonatal DNA methylation associated with two-year attention problems in children born very preterm
Translational Psychiatry (2024)
-
Predicting childhood and adolescent attention-deficit/hyperactivity disorder onset: a nationwide deep learning approach
Molecular Psychiatry (2023)
-
DNA methylation is associated with prenatal exposure to sulfur dioxide and childhood attention-deficit hyperactivity disorder symptoms
Scientific Reports (2023)
-
An overview on neurobiology and therapeutics of attention-deficit/hyperactivity disorder
Discover Mental Health (2023)