Abstract
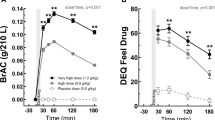
Preclinical evidence suggests that ghrelin, a peptide synthesized by endocrine cells of the stomach and a key component of the gut–brain axis, is involved in alcohol seeking as it modulates both central reward and stress pathways. However, whether and how ghrelin administration may impact alcohol intake in humans is not clear. For, we believe, the first time, this was investigated in the present randomized, crossover, double-blind, placebo-controlled, human laboratory study. Participants were non-treatment-seeking alcohol-dependent heavy-drinking individuals. A 10-min loading dose of intravenous ghrelin/placebo (3 mcg kg−1) followed by a continuous ghrelin/placebo infusion (16.9 ng/kg/min) was administered. During a progressive-ratio alcohol self-administration experiment, participants could press a button to receive intravenous alcohol using the Computerized Alcohol Infusion System. In another experiment, brain functional magnetic resonance imaging was conducted while participants performed a task to gain points for alcohol, food or no reward. Results showed that intravenous ghrelin, compared to placebo, significantly increased the number of alcohol infusions self-administered (percent change: 24.97±10.65, P=0.04, Cohen’s d=0.74). Participants were also significantly faster to initiate alcohol self-administration when they received ghrelin, compared to placebo (P=0.03). The relationships between breath alcohol concentration and subjective effects of alcohol were also moderated by ghrelin administration. Neuroimaging data showed that ghrelin increased the alcohol-related signal in the amygdala (P=0.01) and modulated the food-related signal in the medial orbitofrontal cortex (P=0.01) and nucleus accumbens (P=0.08). These data indicate that ghrelin signaling affects alcohol seeking in humans and should be further investigated as a promising target for developing novel medications for alcohol use disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 2016; 3: 760–773.
Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci 2011; 12: 453–466.
Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev 2005; 85: 495–522.
Howick K, Griffin BT, Cryan JF, Schellekens H. From belly to brain: targeting the ghrelin receptor in appetite and food intake regulation. Int J Mol Sci 2017; 18: E273.
Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res 1997; 48: 23–29.
Jiang H, Betancourt L, Smith RG. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol 2006; 20: 1772–1785.
Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol 2007; 12: 6–16.
Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 2006; 116: 3229–3239.
Weinberg ZY, Nicholson ML, Currie PJ. 6-Hydroxydopamine lesions of the ventral tegmental area suppress ghrelin's ability to elicit food-reinforced behavior. Neurosci Lett 2011; 499: 70–73.
Spencer SJ, Emmerzaal TL, Kozicz T, Andrews ZB. Ghrelin’s role in the hypothalamic-pituitary-adrenal axis stress response: implications for mood disorders. Biol Psychiatry 2015; 78: 19–27.
Hansson C, Alvarez-Crespo M, Taube M, Skibicka KP, Schmidt L, Karlsson-Lindahl L et al. Influence of ghrelin on the central serotonergic signaling system in mice. Neuropharmacology 2014; 79: 498–505.
Landgren S, Engel JA, Hyytia P, Zetterberg H, Blennow K, Jerlhag E. Expression of the gene encoding the ghrelin receptor in rats selected for differential alcohol preference. Behav Brain Res 2011; 221: 182–188.
Szulc M, Mikolajczak PL, Geppert B, Wachowiak R, Dyr W, Bobkiewicz-Kozlowska T. Ethanol affects acylated and total ghrelin levels in peripheral blood of alcohol-dependent rats. Addict Biol 2013; 18: 689–701.
Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict Biol 2008; 13: 358–363.
Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D et al. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci USA 2009; 106: 11318–11323.
Cepko LC, Selva JA, Merfeld EB, Fimmel AI, Goldberg SA, Currie PJ. Ghrelin alters the stimulatory effect of cocaine on ethanol intake following mesolimbic or systemic administration. Neuropharmacology 2014; 85: 224–231.
Bahi A, Tolle V, Fehrentz JA, Brunel L, Martinez J, Tomasetto CL et al. Ghrelin knockout mice show decreased voluntary alcohol consumption and reduced ethanol-induced conditioned place preference. Peptides 2013; 43: 48–55.
Gomez JL, Cunningham CL, Finn DA, Young EA, Helpenstell LK, Schuette LM et al. Differential effects of ghrelin antagonists on alcohol drinking and reinforcement in mouse and rat models of alcohol dependence. Neuropharmacology 2015; 97: 182–193.
Addolorato G, Capristo E, Leggio L, Ferrulli A, Abenavoli L, Malandrino N et al. Relationship between ghrelin levels, alcohol craving, and nutritional status in current alcoholic patients. Alcohol Clin Exp Res 2006; 30: 1933–1937.
Badaoui A, De Saeger C, Duchemin J, Gihousse D, de Timary P, Starkel P. Alcohol dependence is associated with reduced plasma and fundic ghrelin levels. Eur J Clin Invest 2008; 38: 397–403.
de Timary P, Cani PD, Duchemin J, Neyrinck AM, Gihousse D, Laterre PF et al. The loss of metabolic control on alcohol drinking in heavy drinking alcohol-dependent subjects. PLoS One 2012; 7: e38682.
Leggio L, Ferrulli A, Cardone S, Nesci A, Miceli A, Malandrino N et al. Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving. Addict Biol 2012; 17: 452–464.
Koopmann A, von der Goltz C, Grosshans M, Dinter C, Vitale M, Wiedemann K et al. The association of the appetitive peptide acetylated ghrelin with alcohol craving in early abstinent alcohol dependent individuals. Psychoneuroendocrinology 2012; 37: 980–986.
Akkisi Kumsar N, Dilbaz N. Relationship between craving and ghrelin, adiponectin, and resistin levels in patients with alcoholism. Alcohol Clin Exp Res 2015; 39: 702–709.
Kraus T, Schanze A, Groschl M, Bayerlein K, Hillemacher T, Reulbach U et al. Ghrelin levels are increased in alcoholism. Alcohol Clin Exp Res 2005; 29: 2154–2157.
Leggio L, Zywiak WH, Fricchione SR, Edwards SM, de la Monte SM, Swift RM et al. Intravenous ghrelin administration increases alcohol craving in alcohol-dependent heavy drinkers: a preliminary investigation. Biol Psychiatry 2014; 76: 734–741.
Garin MC, Burns CM, Kaul S, Cappola AR. Clinical review: the human experience with ghrelin administration. J Clin Endocrinol Metab 2013; 98: 1826–1837.
Zimmermann US, O'Connor S, Ramchandani VA. Modeling alcohol self-administration in the human laboratory. Curr Top Behav Neurosci 2013; 13: 315–353.
Czachowski CL, Legg BH, Samson HH. Assessment of sucrose and ethanol reinforcement: the across-session breakpoint procedure. Physiol Behav 2003; 78: 51–59.
Plawecki MH, Wetherill L, Vitvitskiy V, Kosobud A, Zimmermann US, Edenberg HJ et al. Voluntary intravenous self-administration of alcohol detects an interaction between GABAergic manipulation and GABRG1 polymorphism genotype: a pilot study. Alcohol Clin Exp Res 2013; 37(Suppl 1): E152–E160.
Vatsalya V, Gowin JL, Schwandt ML, Momenan R, Coe MA, Cooke ME et al. Effects of varenicline on neural correlates of alcohol salience in heavy drinkers. Int J Neuropsychopharmacol 2015; 18: pyv068.
Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 2000; 12: 20–27.
Ramchandani VA, Bolane J, Li TK, O'Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res 1999; 23: 617–623.
Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29: 162–173.
Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 2001; 21: Rc159.
Hommer DW, Knutson B, Fong GW, Bennett S, Adams CM, Varnera JL. Amygdalar recruitment during anticipation of monetary rewards: an event-related fMRI study. Ann NY Acad Sci 2003; 985: 476–478.
Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci 2004; 24: 1793–1802.
Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 2008; 7: 400–409.
Karra E, O'Daly OG, Choudhury AI, Yousseif A, Millership S, Neary MT et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J Clin Invest 2013; 123: 3539–3551.
Goldstone AP, Prechtl CG, Scholtz S, Miras AD, Chhina N, Durighel G et al. Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food. Am J Clin Nutr 2014; 99: 1319–1330.
Kunath N, Muller NC, Tonon M, Konrad BN, Pawlowski M, Kopczak A et al. Ghrelin modulates encoding-related brain function without enhancing memory formation in humans. Neuroimage 2016; 142: 465–473.
Keizer RJ, Jansen RS, Rosing H, Thijssen B, Beijnen JH, Schellens JH et al. Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol Res Perspect 2015; 3: e00131.
Davis JF, Schurdak JD, Magrisso IJ, Mul JD, Grayson BE, Pfluger PT et al. Gastric bypass surgery attenuates ethanol consumption in ethanol-preferring rats. Biol Psychiatry 2012; 72: 354–360.
Ralevski E, Horvath TL, Shanabrough M, Hayden R, Newcomb J, Petrakis I. Ghrelin is supressed by intravenous alcohol and is related to stimulant and sedative effects of alcohol. Alcohol Alcohol 2017; 52: 431–438.
Volkow ND, Wang GJ, Tomasi D, Baler RD. The addictive dimensionality of obesity. Biol Psychiatry 2013; 73: 811–818.
Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001; 50: 1714–1719.
Anderberg RH, Hansson C, Fenander M, Richard JE, Dickson SL, Nissbrandt H et al. The stomach-derived hormone ghrelin increases impulsive behavior. Neuropsychopharmacology 2016; 41: 1199–1209.
Koob GF. The dark side of emotion: the addiction perspective. Eur J Pharmacol 2015; 753: 73–87.
Alvarez-Crespo M, Skibicka KP, Farkas I, Molnar CS, Egecioglu E, Hrabovszky E et al. The amygdala as a neurobiological target for ghrelin in rats: neuroanatomical, electrophysiological and behavioral evidence. PLoS One 2012; 7: e46321.
Song L, Zhu Q, Liu T, Yu M, Xiao K, Kong Q et al. Ghrelin modulates lateral amygdala neuronal firing and blocks acquisition for conditioned taste aversion. PLoS One 2013; 8: e65422.
Hansson C, Haage D, Taube M, Egecioglu E, Salome N, Dickson SL. Central administration of ghrelin alters emotional responses in rats: behavioural, electrophysiological and molecular evidence. Neuroscience 2011; 180: 201–211.
Meyer RM, Burgos-Robles A, Liu E, Correia SS, Goosens KA. A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol Psychiatry 2014; 19: 1284–1294.
Jensen M, Ratner C, Rudenko O, Christiansen SH, Skov LJ, Hundahl C et al. Anxiolytic-like effects of increased ghrelin receptor signaling in the amygdala. Int J Neuropsychopharmacol 2016; 19: pyv123.
Yoshimoto K, Nagao M, Watanabe Y, Yamaguchi T, Ueda S, Kitamura Y et al. Enhanced alcohol-drinking behavior associated with active ghrelinergic and serotoninergic neurons in the lateral hypothalamus and amygdala. Pharmacol Biochem Behav 2017; 153: 1–11.
Cruz MT, Herman MA, Cote DM, Ryabinin AE, Roberto M. Ghrelin increases GABAergic transmission and interacts with ethanol actions in the rat central nucleus of the amygdala. Neuropsychopharmacology 2013; 38: 364–375.
Stalnaker TA, Cooch NK, Schoenbaum G. What the orbitofrontal cortex does not do. Nat Neurosci 2015; 18: 620–627.
Sun X, Veldhuizen MG, Wray AE, de Araujo IE, Sherwin RS, Sinha R et al. The neural signature of satiation is associated with ghrelin response and triglyceride metabolism. Physiol Behav 2014; 136: 63–73.
Holsen LM, Lawson EA, Christensen K, Klibanski A, Goldstein JM. Abnormal relationships between the neural response to high- and low-calorie foods and endogenous acylated ghrelin in women with active and weight-recovered anorexia nervosa. Psychiatry Res 2014; 223: 94–103.
Seo D, Sinha R. The neurobiology of alcohol craving and relapse. Handb Clin Neurol 2014; 125: 355–368.
Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol 2006; 11: 45–54.
Jerlhag E, Janson AC, Waters S, Engel JA. Concomitant release of ventral tegmental acetylcholine and accumbal dopamine by ghrelin in rats. PLoS One 2012; 7: e49557.
Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides 2005; 26: 2274–2279.
Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience 2011; 180: 129–137.
Quarta D, Di Francesco C, Melotto S, Mangiarini L, Heidbreder C, Hedou G. Systemic administration of ghrelin increases extracellular dopamine in the shell but not the core subdivision of the nucleus accumbens. Neurochem Int 2009; 54: 89–94.
van Zessen R, van der Plasse G, Adan RA. Contribution of the mesolimbic dopamine system in mediating the effects of leptin and ghrelin on feeding. Proc Nutr Soc 2012; 71: 435–445.
Sarvari M, Kocsis P, Deli L, Gajari D, David S, Pozsgay Z et al. Ghrelin modulates the fMRI BOLD response of homeostatic and hedonic brain centers regulating energy balance in the rat. PLoS One 2014; 9: e97651.
Gorka SM, Fitzgerald DA, King AC, Phan KL. Alcohol attenuates amygdala-frontal connectivity during processing social signals in heavy social drinkers: a preliminary pharmaco-fMRI study. Psychopharmacology (Berl) 2013; 229: 141–154.
O'Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML et al. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology 2012; 37: 2267–2276.
Peters S, Jolles DJ, Van Duijvenvoorde AC, Crone EA, Peper JS. The link between testosterone and amygdala-orbitofrontal cortex connectivity in adolescent alcohol use. Psychoneuroendocrinology 2015; 53: 117–126.
Peters S, Peper JS, Van Duijvenvoorde AC, Braams BR, Crone EA. Amygdala-orbitofrontal connectivity predicts alcohol use two years later: a longitudinal neuroimaging study on alcohol use in adolescence. Dev Sci 2016; 20: e12448.
Jones RB, McKie S, Astbury N, Little TJ, Tivey S, Lassman DJ et al. Functional neuroimaging demonstrates that ghrelin inhibits the central nervous system response to ingested lipid. Gut 2012; 61: 1543–1551.
Zhang CJ, Bidlingmaier M, Altaye M, Page LC, D'Alessio D, Tschop MH et al. Acute administration of acyl, but not desacyl ghrelin, decreases blood pressure in healthy humans. Eur J Endocrinol 2017; 176: 123–132.
Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther 2002; 302: 822–827.
Tamboli RA, Antoun J, Sidani RM, Clements BA, Eckert EA, Marks-Shulman P et al. Metabolic responses to exogenous ghrelin in obesity and early after Roux-en-Y gastric bypass in humans. Diabetes Obes Metab 2017; 19: 1267–1275.
Kiefer F, Jahn H, Jaschinski M, Holzbach R, Wolf K, Naber D et al. Leptin: a modulator of alcohol craving? Biol Psychiatry 2001; 49: 782–787.
Suchankova P, Yan J, Schwandt ML, Stangl BL, Caparelli EC, Momenan R et al. The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence. Transl Psychiatry 2015; 5: e583.
Tong J, Dave N, Mugundu GM, Davis HW, Gaylinn BD, Thorner MO et al. The pharmacokinetics of acyl, des-acyl, and total ghrelin in healthy human subjects. Eur J Endocrinol 2013; 168: 821–828.
Cui H, Lopez M, Rahmouni K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat Rev Endocrinol 2017; 13: 338–351.
Acknowledgments
We thank the clinical and research staff involved in data collection and support at the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Division of Intramural Clinical and Biological Research, that is, the NIAAA/NIDA Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology and the NIAAA clinical intramural program. We also thank the clinical and research staff involved in data collection, patient care and clinical/technical support at the following NIH Clinical Center Departments: Nursing (in particular, the nurses of the 1SE Inpatient Unit and of the 1-HALC 1SE Outpatient Clinic), Nutrition (in particular LT Kelly Ratteree, MPH, RDN and CDR Merel Kozlosky, MS, RD) and Pharmacy. Furthermore, we thank Ms Karen Smith from the NIH Library for bibliographic assistance. We also thank the deceased and profoundly missed Daniel W. Hommer, MD, for his critical input and guidance during the early phase of development of the fMRI experiment of this protocol. Finally, we would like to express our gratitude to the participants who took part in this study.
This work was supported by National Institutes of Health (NIH) intramural funding ZIA-AA000218 (Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology; PI: Dr Lorenzo Leggio), jointly supported by the Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the Intramural Research Program of the National Institute on Drug Abuse (NIDA). The development of the Computerized Alcohol Infusion System (CAIS) software was supported by the NIAAA-funded Indiana Alcohol Research Center (AA007611).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors report no biomedical financial interests or potential conflicts of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Farokhnia, M., Grodin, E.N., Lee, M.R. et al. Exogenous ghrelin administration increases alcohol self-administration and modulates brain functional activity in heavy-drinking alcohol-dependent individuals. Mol Psychiatry 23, 2029–2038 (2018). https://doi.org/10.1038/mp.2017.226
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.226
This article is cited by
-
Ghrelin decreases sensitivity to negative feedback and increases prediction-error related caudate activity in humans, a randomized controlled trial
Neuropsychopharmacology (2024)
-
Using naltrexone to validate a human laboratory test system to screen new medications for alcoholism (TESMA)- a randomized clinical trial
Translational Psychiatry (2023)
-
G-CuP: the effect of a forced oral glucose intake on alcohol craving and mesolimbic cue reactivity in alcohol dependence—study protocol of a randomized, double-blind, placebo-controlled crossover study
Trials (2022)
-
Involvement of the ghrelin system in the maintenance and reinstatement of cocaine-motivated behaviors: a role of adrenergic action at peripheral β1 receptors
Neuropsychopharmacology (2022)
-
Involvement of the ghrelin system in the maintenance of oxycodone self-administration: converging evidence from endocrine, pharmacologic and transgenic approaches
Molecular Psychiatry (2022)