Abstract
Major depressive disorder (MDD) is a complex and heterogeneous mood disorder, making it difficult to develop a generalized, pharmacological therapy that is effective for all who suffer from MDD. Through the fortuitous discovery of N-methyl-D-aspartate receptor (NMDAR) antagonists as effective antidepressants, we have gained key insights into how antidepressant effects can be produced at the circuit and molecular levels. NMDAR antagonists act as rapid-acting antidepressants such that relief from depressive symptoms occurs within hours of a single injection. The mode of action of NMDAR antagonists seemingly relies on their ability to activate protein-synthesis-dependent homeostatic mechanisms that restore top–down excitatory connections. Recent evidence suggests that NMDAR antagonists relieve depressive symptoms by forming new synapses resulting in increased excitatory drive. This event requires the mammalian target of rapamycin complex 1 (mTORC1), a signaling pathway that regulates synaptic protein synthesis. Herein, we review critical studies that shed light on the action of NMDAR antagonists as rapid-acting antidepressants and how they engage a neuron’s or neural network’s homeostatic mechanisms to self-correct. Recent studies notably demonstrate that a shift in γ-amino-butyric acid receptor B (GABABR) function, from inhibitory to excitatory, is required for mTORC1-dependent translation with NMDAR antagonists. Finally, we discuss how GABABR activation of mTORC1 helps resolve key discrepancies between rapid-acting antidepressants and local homeostatic mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Zarate C Jr., Machado-Vieira R, Henter I, Ibrahim L, Diazgranados N, Salvadore G . Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry 2010; 18: 293–303.
Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC . The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 2015; 76: 155–162.
Luscher B, Shen Q, Sahir N . The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 2011; 16: 383–406.
Bellone C, Nicoll RA . Rapid bidirectional switching of synaptic NMDA receptors. Neuron 2007; 55: 779–785.
Nestler EJ, Hyman SE . Animal models of neuropsychiatric disorders. Nat Neurosci 2010; 13: 1161–1169.
Krishnan V, Nestler EJ . Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry 2010; 167: 1305–1320.
Krishnan V, Nestler EJ . The molecular neurobiology of depression. Nature 2008; 455: 894–902.
Murrough JW, Iacoviello B, Neumeister A, Charney DS, Iosifescu DV . Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol Learn Mem 2011; 96: 553–563.
Murrough JW . Ketamine as a novel antidepressant: from synapse to behavior. Clin Pharmacol Ther 2012; 91: 303–309.
Murrough JW, Charney DS . Is there anything really novel on the antidepressant horizon? Curr Psychiatry Rep 2012; 14: 643–649.
Trivedi MH, Hollander E, Nutt D, Blier P . Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. J Clin Psychiatry 2008; 69: 246–258.
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006; 163: 28–40.
Duman RS, Monteggia LM . A neurotrophic model for stress-related mood disorders. Biol Psychiatry 2006; 59: 1116–1127.
Levinstein MR, Samuels BA . Mechanisms underlying the antidepressant response and treatment resistance. Front Behav Neurosci 2014; 8: 208.
Price JL, Drevets WC . Neurocircuitry of mood disorders. Neuropsychopharmacology 2010; 35: 192–216.
Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology 2017; 42: 1210–1219.
Duman RS . Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues Clin Neurosci 2014; 16: 11–27.
Bearden CE, Thompson PM, Avedissian C, Klunder AD, Nicoletti M, Dierschke N et al. Altered hippocampal morphology in unmedicated patients with major depressive illness. ASN Neuro 2009; 1: 265–273.
Genzel L, Dresler M, Cornu M, Jager E, Konrad B, Adamczyk M et al. Medial prefrontal-hippocampal connectivity and motor memory consolidation in depression and schizophrenia. Biol Psychiatry 2015; 77: 177–186.
Drevets WC, Price JL, Furey ML . Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 2008; 213: 93–118.
Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 2006; 26: 7870–7874.
Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol 2008; 507: 1141–1150.
Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z . Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 2012; 73: 962–977.
Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X . An excitatory synapse hypothesis of depression. Trends Neurosci 2015; 38: 279–294.
Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC . Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 2007; 64: 193–200.
Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, MM P et al. Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol 2008; 11: 255–260.
Chang LC, Jamain S, Lin CW, Rujescu D, Tseng GC, Sibille E . A conserved BDNF, glutamate- and GABA-enriched gene module related to human depression identified by coexpression meta-analysis and DNA variant genome-wide association studies. PLoS ONE 2014; 9: e90980.
Workman ER, Haddick PC, Bush K, Dilly GA, Niere F, Zemelman BV et al. Rapid antidepressants stimulate the decoupling of GABA(B) receptors from GIRK/Kir3 channels through increased protein stability of 14-3-3eta. Mol Psychiatry 2015; 20: 298–310.
Magarinos AM, McEwen BS . Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 1995; 69: 89–98.
McEwen BS, Eiland L, Hunter RG, Miller MM . Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 2012; 62: 3–12.
Pawlak R, Rao BS, Melchor JP, Chattarji S, McEwen B, Strickland S . Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci USA 2005; 102: 18201–18206.
Drevets WC . Neuroplasticity in mood disorders. Dialogues Clin Neurosci 2004; 6: 199–216.
Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 2004; 56: 640–650.
Hu W, Zhang M, Czeh B, Flugge G, Zhang W . Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology 2010; 35: 1693–1707.
Kendler KS, Karkowski LM, Prescott CA . Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 1999; 156: 837–841.
Trullas R, Folio T, Young A, Miller R, Boje K, Skolnick P . 1-aminocyclopropanecarboxylates exhibit antidepressant and anxiolytic actions in animal models. Eur J Pharmacol 1991; 203: 379–385.
Soden ME, Chen L . Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. J Neurosci 2010; 30: 16910–16921.
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016; 533: 481–486.
Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 2013; 38: 729–742.
Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E et al. GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology 2017; 42: 1231–1242.
Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF et al. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs 2014; 23: 243–254.
Zanos P, Nelson ME, Highland JN, Krimmel SR, Georgiou P, Gould TD et al. A negative allosteric modulator for alpha5 subunit-containing GABA receptors exerts a rapid and persistent antidepressant-like action without the side effects of the NMDA receptor antagonist ketamine in mice. eNeuro 2017; 4: 1–11.
Duman RS, Aghajanian GK . Synaptic dysfunction in depression: potential therapeutic targets. Science 2012; 338: 68–72.
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C et al. Deep brain stimulation for treatment-resistant depression. Neuron 2005; 45: 651–660.
Turrigiano G . Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol 2012; 4: a005736.
Turrigiano GG, Nelson SB . Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol 2000; 10: 358–364.
Blanke ML, VanDongen AMJ . Activation mechanisms of the NMDA receptor. In: Van Dongen AM, ed. Biology of the NMDA Receptor. Frontiers in Neuroscience: Boca Raton (FL), 2009.
Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1774–1779.
Paoletti P, Neyton J . NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol 2007; 7: 39–47.
Hunt DL, Castillo PE . Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr Opin Neurobiol 2012; 22: 496–508.
Bloodgood BL, Sabatini BL . NMDA receptor-mediated calcium transients in dendritic spines. In: Van Dongen AM, ed. Biology of the NMDA Receptor. Frontiers in Neuroscience: Boca Raton (FL), 2009.
Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM . Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 2006; 125: 785–799.
Parsons CG, Stoffler A, Danysz W . Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system—too little activation is bad, too much is even worse. Neuropharmacology 2007; 53: 699–723.
Mathews DC, Henter ID, Zarate CA . Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs 2012; 72: 1313–1333.
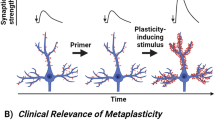
Lee MC, Yasuda R, Ehlers MD . Metaplasticity at single glutamatergic synapses. Neuron 2010; 66: 859–870.
Ibrahim L, Duncan W, Luckenbaugh DA, Yuan P, Machado-Vieira R, Zarate CA Jr . Rapid antidepressant changes with sleep deprivation in major depressive disorder are associated with changes in vascular endothelial growth factor (VEGF): a pilot study. Brain Res Bull 2011; 86: 129–133.
Murrough JW, Abdallah CG, Mathew SJ . Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov 2017; 16: 472–486.
Muller J, Pentyala S, Dilger J, Pentyala S . Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther Adv Psychopharmacol 2016; 6: 185–192.
Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 2015; 172: 950–966.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011; 475: 91–95.
Browne CA, Lucki I . Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol 2013; 4: 161.
Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM . Effects of a ketamine metabolite on synaptic NMDAR function. Nature 2017; 546: E1–E3.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–964.
Workman ER, Niere F, Raab-Graham KF . mTORC1-dependent protein synthesis underlying rapid antidepressant effect requires GABABR signaling. Neuropharmacology 2013; 73: 192–203.
Machado-Vieira R, Henter ID, Zarate CA Jr . New targets for rapid antidepressant action. Prog Neurobiol 2017; 152: 21–37.
Miller OH, Moran JT, Hall BJ . Two cellular hypotheses explaining the initiation of ketamine's antidepressant actions: direct inhibition and disinhibition. Neuropharmacology 2016; 100: 17–26.
Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET . Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci 2013; 33: 6990–7002.
Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife 2014; 3: e03581.
Duman RS, Li N, Liu RJ, Duric V, Aghajanian G . Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 2012; 62: 35–41.
Maeng S, Zarate CA Jr., Du J, Schloesser RJ, McCammon J, Chen G et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 2008; 63: 349–352.
Pozzi L, Pollak Dorocic I, Wang X, Carlen M, Meletis K . Mice lacking NMDA receptors in parvalbumin neurons display normal depression-related behavior and response to antidepressant action of NMDAR antagonists. PLoS ONE 2014; 9: e83879.
Fuchs T, Jefferson SJ, Hooper A, Yee PH, Maguire J, Luscher B . Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol Psychiatry 2017; 22: 920–930.
Povysheva NV, Johnson JW . Tonic NMDA receptor-mediated current in prefrontal cortical pyramidal cells and fast-spiking interneurons. J Neurophysiol 2012; 107: 2232–2243.
Sara Y, Virmani T, Deak F, Liu X, Kavalali ET . An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron 2005; 45: 563–573.
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011; 69: 754–761.
Hoeffer CA, Klann E . mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 2010; 33: 67–75.
Gideons ES, Kavalali ET, Monteggia LM . Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci USA 2014; 111: 8649–8654.
Zhang K, Yamaki VN, Wei Z, Zheng Y, Cai X . Differential regulation of GluA1 expression by ketamine and memantine. Behav Brain Res 2017; 316: 152–159.
Heise C, Gardoni F, Culotta L, di Luca M, Verpelli C, Sala C . Elongation factor-2 phosphorylation in dendrites and the regulation of dendritic mRNA translation in neurons. Front Cell Neurosci 2014; 8: 35.
Hay N, Sonenberg N . Upstream and downstream of mTOR. Genes Dev 2004; 18: 1926–1945.
Hoeffer CA, Klann E . NMDA receptors and translational control. In: Van Dongen AM, ed. Biology of the NMDA Receptor. Frontiers in Neuroscience: Boca Raton (FL), 2009.
Padgett CL, Slesinger PA . GABAB receptor coupling to G-proteins and ion channels. Adv Pharmacol 2010; 58: 123–147.
Chalifoux JR, Carter AG . GABAB receptors modulate NMDA receptor calcium signals in dendritic spines. Neuron 2010; 66: 101–113.
Otmakhova NA, Lisman JE . Contribution of Ih and GABAB to synaptically induced afterhyperpolarizations in CA1: a brake on the NMDA response. J Neurophysiol 2004; 92: 2027–2039.
Huang CS, Shi SH, Ule J, Ruggiu M, Barker LA, Darnell RB et al. Common molecular pathways mediate long-term potentiation of synaptic excitation and slow synaptic inhibition. Cell 2005; 123: 105–118.
Niciu MJ, Ionescu DF, Richards EM, Zarate CA Jr . Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm (Vienna) 2014; 121: 907–924.
Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS . BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 2015; 18: 1.
Sutton MA, Schuman EM . Dendritic protein synthesis, synaptic plasticity, and memory. Cell 2006; 127: 49–58.
Hou Q, Zhang D, Jarzylo L, Huganir RL, Man HY . Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc Natl Acad Sci USA 2008; 105: 775–780.
Thiagarajan TC, Lindskog M, Tsien RW . Adaptation to synaptic inactivity in hippocampal neurons. Neuron 2005; 47: 725–737.
Jakawich SK, Nasser HB, Strong MJ, McCartney AJ, Perez AS, Rakesh N et al. Local presynaptic activity gates homeostatic changes in presynaptic function driven by dendritic BDNF synthesis. Neuron 2010; 68: 1143–1158.
Ibata K, Sun Q, Turrigiano GG . Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron 2008; 57: 819–826.
Quibell R, Prommer EE, Mihalyo M, Twycross R, Wilcock A . Ketamine*. J Pain Symptom Manage 2011; 41: 640–649.
De Simoni A, Griesinger CB, Edwards FA . Development of rat CA1 neurones in acute versus organotypic slices: role of experience in synaptic morphology and activity. J Physiol 2003; 550 (Pt 1): 135–147.
Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ et al. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci. 2010; 13: 1090–1097.
Ivenshitz M, Segal M . Neuronal density determines network connectivity and spontaneous activity in cultured hippocampus. J Neurophysiol 2010; 104: 1052–1060.
Duncan WC Jr., Zarate CA Jr . Ketamine, sleep, and depression: current status and new questions. Curr Psychiatry Rep 2013; 15: 394.
Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G . Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci 2008; 11: 200–208.
Huber R, Ghilardi MF, Massimini M, Tononi G . Local sleep and learning. Nature 2004; 430: 78–81.
Serchov T, Clement HW, Schwarz MK, Iasevoli F, Tosh DK, Idzko M et al. Increased signaling via adenosine A1 receptors, sleep deprivation, imipramine, and ketamine inhibit depressive-like behavior via induction of Homer1a. Neuron 2015; 87: 549–562.
Bus BA, Molendijk ML, Tendolkar I, Penninx BW, Prickaerts J, Elzinga BM et al. Chronic depression is associated with a pronounced decrease in serum brain-derived neurotrophic factor over time. Mol Psychiatry 2015; 20: 602–608.
Gorgulu Y, Caliyurt O . Rapid antidepressant effects of sleep deprivation therapy correlates with serum BDNF changes in major depression. Brain Res Bull 2009; 80: 158–162.
Duncan WC, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS et al. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol 2013; 16: 301–311.
Hoyer C, Kranaster L, Sartorius A, Hellweg R, Gass P . Long-term course of brain-derived neurotrophic factor serum levels in a patient treated with deep brain stimulation of the lateral habenula. Neuropsychobiology 2012; 65: 147–152.
Cryan JF, Kaupmann K . Don't worry 'B' happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci 2005; 26: 36–43.
Cryan JF, Slattery DA . GABAB receptors and depression. Current status. Adv Pharmacol 2010; 58: 427–451.
Pilc A, Lloyd KG . Chronic antidepressants and GABA "B" receptors: a GABA hypothesis of antidepressant drug action. Life Sci 1984; 35: 2149–2154.
Hadamitzky M, Herring A, Keyvani K, Doenlen R, Krugel U, Bosche K et al. Acute systemic rapamycin induces neurobehavioral alterations in rats. Behav Brain Res 2014; 273: 16–22.
Russo E, Leo A, Crupi R, Aiello R, Lippiello P, Spiga R et al. Everolimus improves memory and learning while worsening depressive- and anxiety-like behavior in an animal model of depression. J Psychiatr Res 2016; 78: 1–10.
Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK . Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 2012; 71: 996–1005.
Pastuzyn ED, Shepherd JD . Activity-dependent Arc expression and homeostatic synaptic plasticity are altered in neurons from a mouse model of Angelman syndrome. Front Mol Neurosci 2017; 10.
Wolfe SA, Workman ER, Heaney CF, Niere F, Namjoshi S, Cacheaux LP et al. FMRP regulates an ethanol-dependent shift in GABABR function and expression with rapid antidepressant properties. Nat Commun 2016; 7: 12867.
Acknowledgements
This work was supported by an NIH-NIAAA pilot grant provided by the Integrated Neuroscience Initiative on Alcoholism (KRG), NSF grant IOS-1355158 (KRG) and Department of Defense USAMRMC Award W81XWH-14-10061 (KRG). We thank William Taylor for editing this manuscript. We thank Dr Fadel Zeidan for critical discussion and advice regarding human data and MDD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Workman, E., Niere, F. & Raab-Graham, K. Engaging homeostatic plasticity to treat depression. Mol Psychiatry 23, 26–35 (2018). https://doi.org/10.1038/mp.2017.225
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.225
This article is cited by
-
Retinoid homeostasis in major depressive disorder
Translational Psychiatry (2023)
-
Antidepressant actions of ketamine engage cell-specific translation via eIF4E
Nature (2021)
-
Ketamine—50 years in use: from anesthesia to rapid antidepressant effects and neurobiological mechanisms
Pharmacological Reports (2021)
-
Cerebrospinal fluid neuroplasticity-associated protein levels in patients with psychiatric disorders: a multiplex immunoassay study
Translational Psychiatry (2020)
-
Cortical Excitability and Activation of TrkB Signaling During Rebound Slow Oscillations Are Critical for Rapid Antidepressant Responses
Molecular Neurobiology (2019)