Abstract
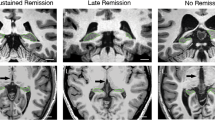
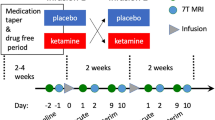
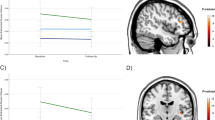
Studies of patients with major depressive disorder (MDD) have consistently reported reduced hippocampal volumes; however, the exact pattern of these volume changes in specific anatomical subfields and their functional significance is unclear. We sought to clarify the relationship between hippocampal tail volumes and (i) a diagnosis of MDD, and (ii) clinical remission to anti-depressant medications (ADMs). Outpatients with nonpsychotic MDD (n=202) based on DSM-IV criteria and a 17-item Hamilton Rating Scale for Depression (HRSD17) score ⩾16 underwent pretreatment magnetic resonance imaging as part of the international Study to Predict Optimized Treatment for Depression (iSPOT-D). Gender-matched healthy controls (n=68) also underwent MRI scanning. An automated pipeline was used to objectively measure hippocampal subfield and whole brain volumes. Remission was defined as an HRSD17 of ⩽7 following 8 weeks of randomized open-label treatment ADMs: escitalopram, sertraline or venlafaxine-extended release. After controlling for age and total brain volume, hippocampal tail volume was larger in the MDD cohort compared to control subjects. Larger hippocampal tail volume was positively related to clinical remission, independent of total hippocampal volume, total brain volume and age. These data provide convergent evidence of the importance of the hippocampus in the development or treatment of MDD. Hippocampal tail volume is proposed as a potentially useful biomarker of sensitivity to ADM treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Arnone D, McIntosh AM, Ebmeier KP, Munafo MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur neuropsychopharmacol 2012; 22: 1–16.
McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J psychiatry neurosci 2009; 34: 41–54.
Maller JJ, Daskalakis ZJ, Fitzgerald PB. Hippocampal volumetrics in depression: the importance of the posterior tail. Hippocampus 2007; 17: 1023–1027.
Malykhin NV, Lebel RM, Coupland NJ, Wilman AH, Carter R. In vivo quantification of hippocampal subfields using 4.7T fast spin echo imaging. NeuroImage 2010; 49: 1224–1230.
Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T et al. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol psychiatry 2005; 57: 935–937.
Malchow B, Strocka S, Frank F, Bernstein HG, Steiner J, Schneider-Axmann T et al. Stereological investigation of the posterior hippocampus in affective disorders. J neural transmission 2015; 122: 1019–1033.
Robinson JL, Barron DS, Kirby LA, Bottenhorn KL, Hill AC, Murphy JE et al. Neurofunctional topography of the human hippocampus. Hum Brain Mapp 2015; 36: 5018–5037.
Cho ZH, Kim YB, Han JY, Kim NB, Hwang SI, Kim SJ et al. Altered T2* relaxation time of the hippocampus in major depressive disorder: implications of ultra-high field magnetic resonance imaging. J psychiatr res 2010; 44: 881–886.
Cole J, Toga AW, Hojatkashani C, Thompson P, Costafreda SG, Cleare AJ et al. Subregional hippocampal deformations in major depressive disorder. J affect disord 2010; 126: 272–277.
Huang Y, Coupland NJ, Lebel RM, Carter R, Seres P, Wilman AH et al. Structural changes in hippocampal subfields in major depressive disorder: a high-field magnetic resonance imaging study. Biol psychiatry 2013; 74: 62–68.
Lindqvist D, Mueller S, Mellon SH, Su Y, Epel ES, Reus VI et al. Peripheral antioxidant markers are associated with total hippocampal and CA3/dentate gyrus volume in MDD and healthy controls-preliminary findings. Psychiatry res 2014; 224: 168–174.
Travis S, Coupland NJ, Silversone PH, Huang Y, Fujiwara E, Carter R et al. Dentate gyrus volume and memory performance in major depressive disorder. J affect disord 2015; 172: 159–164.
Wisse LE, Biessels GJ, Stegenga BT, Kooistra M, van der Veen PH, Zwanenburg JJ et al. Major depressive episodes over the course of 7 years and hippocampal subfield volumes at 7 tesla MRI: the PREDICT-MR study. J affect disord 2015; 175: 1–7.
Travis SG, Coupland NJ, Hegadoren K, Silverstone PH, Huang Y, Carter R et al. Effects of cortisol on hippocampal subfields volumes and memory performance in healthy control subjects and patients with major depressive disorder. J affect disord 2016; 201: 34–41.
Na KS, Chang HS, Won E, Han KM, Choi S, Tae WS et al. Association between glucocorticoid receptor methylation and hippocampal subfields in major depressive disorder. PloS one 2014; 9: e85425.
Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MT, Chakravarty MM et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol psychiatry 2015; 77: 285–294.
Maller JJ, Thaveenthiran P, Thomson RH, McQueen S, Fitzgerald PB. Volumetric, cortical thickness and white matter integrity alterations in bipolar disorder type I and II. J affect disord 2014; 169: 118–127.
Schoene-Bake JC, Keller SS, Niehusmann P, Volmering E, Elger C, Deppe M et al. In vivo mapping of hippocampal subfields in mesial temporal lobe epilepsy: relation to histopathology. Hum Brain Mapp 2014; 35: 4718–4728.
Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. NeuroImage 2015; 115: 117–137.
Iglesias JE, Sabuncu MR, Van Leemput K, Alzheimer's Disease Neuroimaging I. Improved inference in Bayesian segmentation using Monte Carlo sampling: application to hippocampal subfield volumetry. Med image anal 2013; 17: 766–778.
Whelan CD, Hibar DP, van Velzen LS, Zannas AS, Carrillo-Roa T, McMahon K et al. Heritability and reliability of automatically segmented human hippocampal formation subregions. NeuroImage 2016; 128: 125–137.
Han KM, Won E, Sim Y, Tae WS. Hippocampal subfield analysis in medication-naive female patients with major depressive disorder. J affect disord 2016; 194: 21–29.
Phillips JL, Batten LA, Tremblay P, Aldosary F, Blier P. A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment-resistant depression. int j neuropsychopharmacol 2015; 18: 1–9.
Chi KF, Korgaonkar M, Grieve SM. Imaging predictors of remission to anti-depressant medications in major depressive disorder. J affect disord 2015; 186: 134–144.
Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch gen psychiatry 2011; 68: 675–690.
Stratmann M, Konrad C, Kugel H, Krug A, Schoning S, Ohrmann P et al. Insular and hippocampal gray matter volume reductions in patients with major depressive disorder. PloS one 2014; 9: e102692.
MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol psychiatry 2008; 64: 880–883.
Williams LM, Rush AJ, Koslow SH, Wisniewski SR, Cooper NJ, Nemeroff CB et al. International Study to Predict Optimized Treatment for Depression (iSPOT-D), a randomized clinical trial: rationale and protocol. Trials 2011; 12: 4.
Grieve SM, Korgaonkar MS, Etkin A, Harris A, Koslow SH, Wisniewski S et al. Brain imaging predictors and the international study to predict optimized treatment for depression: study protocol for a randomized controlled trial. Trials 2013; 14: 224.
Palacios J, Pazos A, Hoyer D Characterisation and mapping of 5-HT1A receptors in animals and in man. In: Dourish C, Ahlenius S, Hutson P (eds). Brain 5-HT1A Receptors—Behavioural and Neurochemical Pharmacology. Ellis Horwood: Chichester, 1987.
Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain—III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience 1987; 21: 97–122.
Soriano-Mas C, Hernandez-Ribas R, Pujol J, Urretavizcaya M, Deus J, Harrison BJ et al. Cross-sectional and longitudinal assessment of structural brain alterations in melancholic depression. Biol psychiatry 2011; 69: 318–325.
Maller JJ, Daskalakis ZJ, Thomson RH, Daigle M, Barr MS, Fitzgerald PB. Hippocampal volumetrics in treatment-resistant depression and schizophrenia: the devil's in de-tail. Hippocampus 2012; 22: 9–16.
MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA 2003; 100: 1387–1392.
Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. Widespread reductions in gray matter volume in depression. Neuroimage Clin 2013; 3: 332–339.
Miskowiak KW, Vinberg M, Macoveanu J, Ehrenreich H, Koster N, Inkster B et al. Effects of erythropoietin on hippocampal volume and memory in mood disorders. Biol psychiatry 2015; 78: 270–277.
Cao B, Passos IC, Mwangi B, Amaral-Silva H, Tannous J, Wu MJ et al. Hippocampal subfield volumes in mood disorders. Mol Psychiatry 2017; 22: 1352–1358.
Depino AM, Lucchina L, Pitossi F. Early and adult hippocampal TGF-beta1 overexpression have opposite effects on behavior. Brain behav immun 2011; 25: 1582–1591.
Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J psychiatric res 2008; 42: 151–157.
Foster JK, Meikle A, Goodson G, Mayes AR, Howard M, Sunram SI et al. The hippocampus and delayed recall: bigger is not necessarily better? Memory 1999; 7: 715–732.
Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia 2004; 42: 1394–1413.
Malykhin NV, Coupland NJ. Hippocampal neuroplasticity in major depressive disorder. Neuroscience 2015; 309: 200–213.
Wager-Smith K, Markou A. Depression: a repair response to stress-induced neuronal microdamage that can grade into a chronic neuroinflammatory condition? Neurosci biobehav rev 2011; 35: 742–764.
Joca SR, Padovan CM, Guimaraes FS. Activation of post-synaptic 5-HT(1A) receptors in the dorsal hippocampus prevents learned helplessness development. Brain res 2003; 978: 177–184.
Korgaonkar MS, Williams LM, Song YJ, Usherwood T, Grieve SM. Diffusion tensor imaging predictors of treatment outcomes in major depressive disorder. Br j psychiatry 2014; 205: 321–328.
Dunn EC, Brown RC, Dai Y, Rosand J, Nugent NR, Amstadter AB et al. Genetic determinants of depression: recent findings and future directions. Harvard rev psychiatry 2015; 23: 1–18.
Eker MC, Kitis O, Okur H, Eker OD, Ozan E, Isikli S et al. Smaller hippocampus volume is associated with short variant of 5-HTTLPR polymorphism in medication-free major depressive disorder patients. Neuropsychobiology 2011; 63: 22–28.
Grieve SM, Korgaonkar MS, Gordon E, Williams LM, Rush AJ. Prediction of nonremission to antidepressant therapy using diffusion tensor imaging. J Clin Psychiatry 2016; 77: e436–e443.
Acknowledgments
We thank all the patients and volunteers who agreed to participate in this study, and the staff of the MRI facility at the Westmead Hospital, NSW, Australia. Professor Evian Gordon and Lea Williams are acknowledged for their contribution to the instigation and leadership of this project. Dr Mayuresh Korgoankar is thanked for his contribution to data collection and patient recruitment. We acknowledge Brain Resource as the sponsor for the iSPOT-D study (NCT00693849). Claire Day and Catherine King (Global Study Co-ordinators) are thanked as is the iSPOT-D Publication Team for their valuable input into this manuscript and to the study overall. We acknowledge the hard work of the Brain Dynamics Centre iSPOT-D team at the Sydney site for their help with data collection of the presented cohort. Dr Anthony Harris is thanked for his role in clinical supervision of clinical imaging evaluations (as PI for the Sydney site), and Dr Tim Usherwood for his role in overseeing the partnership with primary care practitioners and recruitment of patients from these primary care settings (as co-PI for the Sydney site). Dr Lavier Gomes, Ms Sheryl Foster and the Department of Radiology at Westmead are thanked for their substantial contributions to MRI data acquisition. SMG acknowledges the support of the Sydney Medical School, the Heart Research Institute, the Frecker Family Trust and the Parker-Hughes Bequest.
Brain Resource Ltd is the sponsor for the iSPOT-D study (NCT00693849).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
EG is the CEO of Brain Resource Ltd and has significant equity and stock options in the company. AJR has received consulting fees from Brain Resource Ltd, Duke-NUS, Eli Lilly, Emmes Corp, Liva-Nova Inc., Medavante Inc., Otsuka Pharmaceuticals USA, Santium Inc., Sunovion, Takeda USA, University of Texas Southwestern Med Cntr.; royalties from Guilford Publications and the University of Texas Southwestern Medical Center at Dallas. SK serves on the Board of Directors of MyBrainSolutions and receive compensation in cash and stock options; serve as a private consultant for grant mentoring and preparation to staff at the University of Miami, Miller School of Medicine, Miami, Florida, and Louisiana State University Health Science Center; serves as a Director for the BRAINnet Database. SMG has previously received fees as a consultant for Brain Resource Ltd. The remaining authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Maller, J.J., Broadhouse, K., Rush, A.J. et al. Increased hippocampal tail volume predicts depression status and remission to anti-depressant medications in major depression. Mol Psychiatry 23, 1737–1744 (2018). https://doi.org/10.1038/mp.2017.224
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.224
This article is cited by
-
Reduced gray matter volume of the hippocampal tail in melancholic depression: evidence from an MRI study
BMC Psychiatry (2024)
-
The association between gray matter volume in the hippocampal subfield and antidepressant efficacy mediated by abnormal dynamic functional connectivity
Scientific Reports (2024)
-
Accelerated TMS - moving quickly into the future of depression treatment
Neuropsychopharmacology (2024)
-
Prediction of early improvement of major depressive disorder to antidepressant medication in adolescents with radiomics analysis after ComBat harmonization based on multiscale structural MRI
BMC Psychiatry (2023)
-
Environmental neuroscience linking exposome to brain structure and function underlying cognition and behavior
Molecular Psychiatry (2023)