Abstract
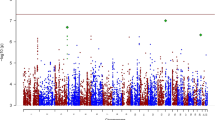
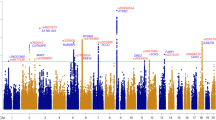
The molecular genetics of panic disorder (PD) with and without agoraphobia (AG) are still largely unknown and progress is hampered by small sample sizes. We therefore performed a genome-wide association study with a dimensional, PD/AG-related anxiety phenotype based on the Agoraphobia Cognition Questionnaire (ACQ) in a sample of 1370 healthy German volunteers of the CRC TRR58 MEGA study wave 1. A genome-wide significant association was found between ACQ and single non-coding nucleotide variants of the GLRB gene (rs78726293, P=3.3 × 10−8; rs191260602, P=3.9 × 10−8). We followed up on this finding in a larger dimensional ACQ sample (N=2547) and in independent samples with a dichotomous AG phenotype based on the Symptoms Checklist (SCL-90; N=3845) and a case–control sample with the categorical phenotype PD/AG (Ncombined =1012) obtaining highly significant P-values also for GLRB single-nucleotide variants rs17035816 (P=3.8 × 10−4) and rs7688285 (P=7.6 × 10−5). GLRB gene expression was found to be modulated by rs7688285 in brain tissue, as well as cell culture. Analyses of intermediate PD/AG phenotypes demonstrated increased startle reflex and increased fear network, as well as general sensory activation by GLRB risk gene variants rs78726293, rs191260602, rs17035816 and rs7688285. Partial Glrb knockout mice demonstrated an agoraphobic phenotype. In conjunction with the clinical observation that rare coding GLRB gene mutations are associated with the neurological disorder hyperekplexia characterized by a generalized startle reaction and agoraphobic behavior, our data provide evidence that non-coding, although functional GLRB gene polymorphisms may predispose to PD by increasing startle response and agoraphobic cognitions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jonsson B et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 2011; 21: 655–679.
Hofmann SG, Alpers GW, Pauli P . Phenomenology of Panic and Phobic Disorders. Oxford University Press: New York, NY, USA, 2008.
Walz N, Mühlberger A, Pauli P . A human open field test reveals thigmotaxis related to agoraphobic fear. Biol Psychiatry 2016; 80: 390–397.
Pauli P, Amrhein C, Muhlberger A, Dengler W, Wiedemann G . Electrocortical evidence for an early abnormal processing of panic-related words in panic disorder patients. Int J Psychophysiol 2005; 57: 33–41.
Chambless DL, Caputo GC, Bright P, Gallagher R . Assessment of fear of fear in agoraphobics: the body sensations questionnaire and the agoraphobic cognitions questionnaire. J Consult Clin Psychol 1984; 52: 1090–1097.
Fyer AJ, Mannuzza S, Chapman TF, Martin LY, Klein DF . Specificity in familial aggregation of phobic disorders. Arch Gen Psychiatry 1995; 52: 564–573.
Noyes R Jr., Crowe RR, Harris EL, Hamra BJ, McChesney CM, Chaudhry DR . Relationship between panic disorder and agoraphobia. A family study. Arch Gen Psychiatry 1986; 43: 227–232.
Mosing MA, Gordon SD, Medland SE, Statham DJ, Nelson EC, Heath AC et al. Genetic and environmental influences on the co-morbidity between depression, panic disorder, agoraphobia, and social phobia: a twin study. Depress Anxiety 2009; 26: 1004–1011.
Gottschalk MG, Domschke K . Novel developments in genetic and epigenetic mechanisms of anxiety. Curr Opin Psychiatry 2016; 29: 32–38.
Hamilton SP . Linkage and association studies of anxiety disorders. Depress Anxiety 2009; 26: 976–983.
Howe AS, Buttenschon HN, Bani-Fatemi A, Maron E, Otowa T, Erhardt A et al. Candidate genes in panic disorder: meta-analyses of 23 common variants in major anxiogenic pathways. Mol Psychiatry 2015; 21: 665–679.
Maron E, Hettema JM, Shlik J . Advances in molecular genetics of panic disorder. Mol Psychiatry 2010; 15: 681–701.
Domschke K, Freitag CM, Kuhlenbaumer G, Schirmacher A, Sand P, Nyhuis P et al. Association of the functional V158M catechol-O-methyl-transferase polymorphism with panic disorder in women. Int J Neuropsychopharmacol 2004; 7: 183–188.
Hamilton SP, Slager SL, Heiman GA, Deng Z, Haghighi F, Klein DF et al. Evidence for a susceptibility locus for panic disorder near the catechol-O-methyltransferase gene on chromosome 22. Biol Psychiatry 2002; 51: 591–601.
Rothe C, Koszycki D, Bradwejn J, King N, Deluca V, Tharmalingam S et al. Association of the Val158Met catechol O-methyltransferase genetic polymorphism with panic disorder. Neuropsychopharmacology 2006; 31: 2237–2242.
Domschke K, Reif A, Weber H, Richter J, Hohoff C, Ohrmann P et al. Neuropeptide S receptor gene — converging evidence for a role in panic disorder. Mol Psychiatry 2010; 16: 938–948.
Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D et al. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet 1999; 8: 621–624.
Domschke K, Tidow N, Kuithan H, Schwarte K, Klauke B, Ambree O et al. Monoamine oxidase A gene DNA hypomethylation - a risk factor for panic disorder? Int J Neuropsychopharmacol 2012; 15: 1217–1228.
Reif A, Richter J, Straube B, Hofler M, Lueken U, Gloster AT et al. MAOA and mechanisms of panic disorder revisited: from bench to molecular psychotherapy. Mol Psychiatry 2014; 19: 122–128.
Domschke K, Deckert J, O'Donovan M C, Glatt SJ . Meta-analysis of COMT val158met in panic disorder: ethnic heterogeneity and gender specificity. Am J Med Genet B Neuropsychiatric Genet 2007; 144B: 667–673.
Reif A, Weber H, Domschke K, Klauke B, Baumann C, Jacob CP et al. Meta-analysis argues for a female-specific role of MAOA-uVNTR in panic disorder in four European populations. Am Med Genet B Neuropsychiatric Genet 2012; 159B: 786–793.
Erhardt A, Czibere L, Roeske D, Lucae S, Unschuld PG, Ripke S et al. TMEM132D, a new candidate for anxiety phenotypes: evidence from human and mouse studies. Mol Psychiatry 2011; 16: 647–663.
Otowa T, Kawamura Y, Nishida N, Sugaya N, Koike A, Yoshida E et al. Meta-analysis of genome-wide association studies for panic disorder in the Japanese population. Transl Psychiatry 2012; 2: e186.
Otowa T, Yoshida E, Sugaya N, Yasuda S, Nishimura Y, Inoue K et al. Genome-wide association study of panic disorder in the Japanese population. J Hum Genet 2009; 54: 122–126.
Erhardt A, Akula N, Schumacher J, Czamara D, Karbalai N, Muller-Myhsok B et al. Replication and meta-analysis of TMEM132D gene variants in panic disorder. Transl Psychiatry 2012; 2: e156.
Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511: 421–427.
Fullerton JM, Willis-Owen SA, Yalcin B, Shifman S, Copley RR, Miller SR et al. Human-mouse quantitative trait locus concordance and the dissection of a human neuroticism locus. Biol Psychiatry 2008; 63: 874–883.
Shifman S, Bhomra A, Smiley S, Wray NR, James MR, Martin NG et al. A whole genome association study of neuroticism using DNA pooling. Mol Psychiatry 2008; 13: 302–312.
Walter S, Glymour MM, Koenen K, Liang L, Tchetgen Tchetgen EJ, Cornelis M et al. Performance of polygenic scores for predicting phobic anxiety. PloS One 2013; 8: e80326.
Otowa T, Hek K, Lee M, Byrne EM, Mirza SS, Nivard MG et al. Meta-analysis of genome-wide association studies of anxiety disorders. Mol Psychiatry 2016; 21: 1485.
Otowa T, Maher BS, Aggen SH, McClay JL, van den Oord EJ, Hettema JM . Genome-wide and gene-based association studies of anxiety disorders in European and African American samples. PloS One 2014; 9: e112559.
Ehlers A, Margraf J Fragebogen zu körperbezogenen Ängsten, Kognitionen und Vermeidung (AKV). Beltz Test 2001; Göttingen.
Domschke K, Gajewska A, Winter B, Herrmann MJ, Warrings B, Muhlberger A et al. ADORA2A Gene variation, caffeine, and emotional processing: a multi-level interaction on startle reflex. Neuropsychopharmacology 2012; 37: 759–769.
Schiele MA, Ziegler C, Holitschke K, Schartner C, Schmidt B, Weber H et al. Influence of 5-HTT variation, childhood trauma and self-efficacy on anxiety traits: a gene-environment-coping interaction study. J Neural Transmission 2016; 123: 895–904.
Hoogendoorn EH, Hermus AR, de Vegt F, Ross HA, Verbeek AL, Kiemeney LA et al. Thyroid function and prevalence of anti-thyroperoxidase antibodies in a population with borderline sufficient iodine intake: influences of age and sex. Clin Chemistry 2006; 52: 104–111.
Derogatis LR, Cleary PA . Factorial invariance across gender for the primary symptom dimensions of the SCL-90. Br J Social Clin Psychol 1977; 16: 347–356.
Derogatis LR, Lipman RS, Covi L . SCL-90: an outpatient psychiatric rating scale—preliminary report. Psychopharmacol Bull 1973; 9: 13–28.
Arrindell WA, Ettema JHM . SCL-90, Handleiding bij een Multidimensionele Psychopathologie Indicator. Swets & Zeitlinger: Lisse, The Netherlands, 1986.
Gloster AT, Wittchen HU, Einsle F, Hofler M, Lang T, Helbig-Lang S et al. Mechanism of action in CBT (MAC): methods of a multi-center randomized controlled trial in 369 patients with panic disorder and agoraphobia. Eur Arch Psychiatry Clin Neurosci 2009; 259 (Suppl 2): S155–S166.
Weber H, Richter J, Straube B, Lueken U, Domschke K, Schartner C et al. Allelic variation in CRHR1 predisposes to panic disorder: evidence for biased fear processing. Mol Psychiatry 2015; 21: 813–822.
Rietschel M, Mattheisen M, Degenhardt F, Genetic R, Outcome in P, Muhleisen TW et al. Association between genetic variation in a region on chromosome 11 and schizophrenia in large samples from Europe. Mol Psychiatry 2012; 17: 906–917.
Fuchsberger C, Abecasis GR, Hinds DA . minimac2: faster genotype imputation. Bioinformatics 2015; 31: 782–784.
Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR . MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol 2010; 34: 816–834.
Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM et al. A global reference for human genetic variation. Nature 2015; 526: 68–74.
Weber H, Klamer D, Freudenberg F, Kittel-Schneider S, Rivero O, Scholz CJ et al. The genetic contribution of the NO system at the glutamatergic post-synapse to schizophrenia: further evidence and meta-analysis. Eur Neuropsychopharmacol 2013; 24: 65–85.
Glotzbach-Schoon E, Andreatta M, Reif A, Ewald H, Troger C, Baumann C et al. Contextual fear conditioning in virtual reality is affected by 5HTTLPR and NPSR1 polymorphisms: effects on fear-potentiated startle. Front Behav Neurosci 2013; 7: 31.
Richter J, Hamm AO, Pane-Farre CA, Gerlach AL, Gloster AT, Wittchen HU et al. Dynamics of defensive reactivity in patients with panic disorder and agoraphobia: implications for the etiology of panic disorder. Biol Psychiatry 2012; 72: 512–520.
Scharfenort R, Lonsdorf TB . Neural correlates of and processes underlying generalized and differential return of fear. Soc Cogn Affect Neurosci 2015; 11: 612–620.
Boucsein W, Fowles DC, Grimnes S, Ben-Shakhar G, Roth WT, Dawson ME et al. Publication recommendations for electrodermal measurements. Psychophysiology 2012; 49: 1017–1034.
Becker K, Braune M, Benderska N, Buratti E, Baralle F, Villmann C et al. A retroelement modifies pre-mRNA splicing: the murine Glrb(spa) allele is a splicing signal polymorphism amplified by long interspersed nuclear element insertion. J Biol Chem 2012; 287: 31185–31194.
Kingsmore SF, Giros B, Suh D, Bieniarz M, Caron MG, Seldin MF . Glycine receptor beta-subunit gene mutation in spastic mouse associated with LINE-1 element insertion. Nat Genet 1994; 7: 136–141.
Crawley JN, Paylor R . A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Hormones Behav 1997; 31: 197–211.
Cuthbert BN, Insel TR . Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 2013; 11: 126.
Hamm AO, Richter J, Pane-Farre C, Westphal D, Wittchen HU, Vossbeck-Elsebusch AN et al. Panic disorder with agoraphobia from a behavioral neuroscience perspective: applying the research principles formulated by the Research Domain Criteria (RDoC) initiative. Psychophysiology 2016; 53: 312–322.
McNally RJ . Anxiety sensitivity and panic disorder. Biol Psychiatry 2002; 52: 938–946.
Godemann F, Schabowska A, Naetebusch B, Heinz A, Strohle A . The impact of cognitions on the development of panic and somatoform disorders: a prospective study in patients with vestibular neuritis. Psychol Med 2006; 36: 99–108.
Kaabi B, Gelernter J, Woods SW, Goddard A, Page GP, Elston RC . Genome scan for loci predisposing to anxiety disorders using a novel multivariate approach: strong evidence for a chromosome 4 risk locus. Am J Hum Genet 2006; 78: 543–553.
Lynch JW . Molecular structure and function of the glycine receptor chloride channel. Physiol Rev 2004; 84: 1051–1095.
Schaefer N, Langlhofer G, Kluck CJ, Villmann C . Glycine receptor mouse mutants: model systems for human hyperekplexia. Br J Pharmacol 2013; 170: 933–952.
Chung SK, Bode A, Cushion TD, Thomas RH, Hunt C, Wood SE et al. GLRB is the third major gene of effect in hyperekplexia. Hum Mol Genet 2013; 22: 927–940.
James VM, Bode A, Chung SK, Gill JL, Nielsen M, Cowan FM et al. Novel missense mutations in the glycine receptor beta subunit gene (GLRB) in startle disease. Neurobiol Dis 2013; 52: 137–149.
Becker CM . Disorders of the inhibitory glycine receptor: the spastic mouse. FASEB J 1990; 4: 2767–2774.
Al-Owain M, Colak D, Al-Bakheet A, Al-Hashmi N, Shuaib T, Al-Hemidan A et al. Novel mutation in GLRB in a large family with hereditary hyperekplexia. Clin Genet 2012; 81: 479–484.
Tijssen MAJ, Rees MI . Hyperekplexia. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH et al (eds). GeneReviews(R). University of Washington: Seattle, WA, USA, 2012.
Anokhin AP, Golosheykin S, Heath AC . Genetic and environmental influences on emotion-modulated startle reflex: a twin study. Psychophysiology 2007; 44: 106–112.
Vaidyanathan U, Malone SM, Miller MB, McGue M, Iacono WG . Heritability and molecular genetic basis of acoustic startle eye blink and affectively modulated startle response: a genome-wide association study. Psychophysiology 2014; 51: 1285–1299.
Davis M, Walker DL, Miles L, Grillon C . Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 2010; 35: 105–135.
Li P, Slaughter M . Glycine receptor subunit composition alters the action of GABA antagonists. Vis Neurosci 2007; 24: 513–521.
Aroeira RI, Ribeiro JA, Sebastiao AM, Valente CA . Age-related changes of glycine receptor at the rat hippocampus: from the embryo to the adult. J Neurochem 2011; 118: 339–353.
Jonsson S, Morud J, Pickering C, Adermark L, Ericson M, Soderpalm B . Changes in glycine receptor subunit expression in forebrain regions of the Wistar rat over development. Brain Res 2012; 1446: 12–21.
Goldman D, Domschke K . Making sense of deep sequencing. Int J Neuropsychopharmacol 2014; 17: 1717–1725.
Acknowledgements
We are grateful to all individuals who participated in this study either as part of the CRC-TRR-58 Mega study wave 1 and 2 or the German PanicNet wave 1 (multicenter trial 'Mechanisms of Action in CBT (MAC)') or wave 2 studies. The study was supported by the DFG CRC-TRR-58 grants Z02 to JD, AR, MR and PP, A08 to RB, B01 to AM and PP, B03 to CB, B06 to AR and CB, B07 to TL, and C02 to KD and JD. RK was supported by the DFG grant KA1623/3-1 and MA by the RTG1253. The MAC study was funded by the German Federal Ministry of Education and Research (BMBF; project no. 01GV0615) as part of the BMBF Psychotherapy Research Funding Initiative. The principal investigators (PIs) of the centers with respective areas of responsibility in the MAC study are: V Arolt (Münster: Overall MAC Program Coordination), H-U Wittchen (Dresden: PI for the Randomized Clinical Trial and Manual Development), A Hamm (Greifswald: PI for Psychophysiology), AL Gerlach (Münster: PI for Psychophysiology and Panic subtypes), A Ströhle (Berlin: PI for Experimental Pharmacology), T Kircher (Marburg: PI for functional neuroimaging) and J Deckert (Würzburg: PI for Genetics). Additional site directors in the randomized controlled trial component of the program are as follows: GW Alpers (Würzburg), T Fydrich and L Fehm (Berlin-Adlershof) and T Lang (Bremen). Further support was obtained from the IZKF-Würzburg Z-6 to HW and the IZKF-Würzburg N-258 to LH. The Nijmegen Biomedical Study is a population-based survey conducted at the Department for Health Evidence and the Department of Laboratory Medicine of the Radboud university medical center. Principal investigators of the Nijmegen Biomedical Study are LALM Kiemeney, ALM Verbeek, DWSwinkels and B Franke. The Genome of the Netherlands Project (http://www.bbmriwiki.nl/wiki/Impute2Pipeline), and specifically Freerk van Dijk and Morris Swertz, are thanked for imputation of the genotype data with 1000 genomes phase 1 integrated version 3 and GoNL4. We credit the MRC Sudden Death Brain and Tissue Bank, Edinburgh, Scotland for providing brain tissue samples examined in this study. T Töpner, I Reck, N Steigerwald, C Gagel and J Auer are credited for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Over the last 3 years, V Arolt has been a member of the advisory boards and/or gave presentations for the following companies: Astra-Zeneca, Eli Lilly, Janssen-Organon, Lundbeck, Otsuka, Servier and Trommsdorff. He also received sponsorships for symposia and educational activities from Astra-Zeneca, Jansen-Organon, Lundbeck and Servier. C Büchel received speaker's honoraria from Janssen. K Domschke has received a honorarium for a scientific talk from Hexal. T Kircher received fees for educational programs from Janssen, Eli Lilly, Servier, Lundbeck, Bristol Myers Squibb, Pfizer and Astra-Zeneca. P Pauli and A Mühlberger are shareholders of a commercial company that develops virtual environment research systems for empirical studies in the field of psychology, psychiatry and psychotherapy. A Ströhle received research funding from Lundbeck, and speaker honoraria from Astra-Zeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly & Co, Lundbeck, Pfizer, Wyeth and UCB. He was a consultant for Actelion. Educational grants were given by the Boehringer Ingelheim Fonds, the Eli Lilly International Foundation, Janssen-Cilag, Pfizer and Eli Lilly & Co. H-U Wittchen has served as a general consultant (non-product related) for Pfizer, Lundbeck, Organon, Servier and Essex Pharma and has received grant funding for his institution from Sanofi Aventis, Pfizer, Lundbeck, Novatis, Essex Pharma, Servier and Wyeth. These cooperations have no relevance to the work that is covered in the manuscript. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Rights and permissions
About this article
Cite this article
Deckert, J., Weber, H., Villmann, C. et al. GLRB allelic variation associated with agoraphobic cognitions, increased startle response and fear network activation: a potential neurogenetic pathway to panic disorder. Mol Psychiatry 22, 1431–1439 (2017). https://doi.org/10.1038/mp.2017.2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.2
This article is cited by
-
Comprehensive behavioral analyses of mice with a glycine receptor alpha 4 deficiency
Molecular Brain (2023)
-
Genetik und Epigenetik von Angsterkrankungen
BIOspektrum (2020)
-
Genetic and epigenetic analyses of panic disorder in the post-GWAS era
Journal of Neural Transmission (2020)
-
Genetics of Anxiety Disorders
Current Psychiatry Reports (2019)
-
Orexin in the anxiety spectrum: association of a HCRTR1 polymorphism with panic disorder/agoraphobia, CBT treatment response and fear-related intermediate phenotypes
Translational Psychiatry (2019)